J Vet Sci.
2013 Dec;14(4):413-419. 10.4142/jvs.2013.14.4.413.
Development and standardization of a monoclonal antibody-based rapid flow-through immunoassay for the detection of Aphanomyces invadans in the field
- Affiliations
-
- 1Aquatic Health Management Laboratory, Department of Aquaculture, College of Fisheries, Karnataka Veterinary, Animal and Fisheries Sciences University, Mangalore 575002, India. kalkulishankar@gmail.com
- KMID: 1712307
- DOI: http://doi.org/10.4142/jvs.2013.14.4.413
Abstract
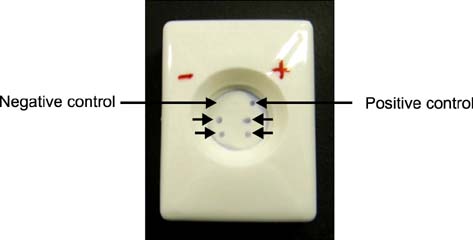
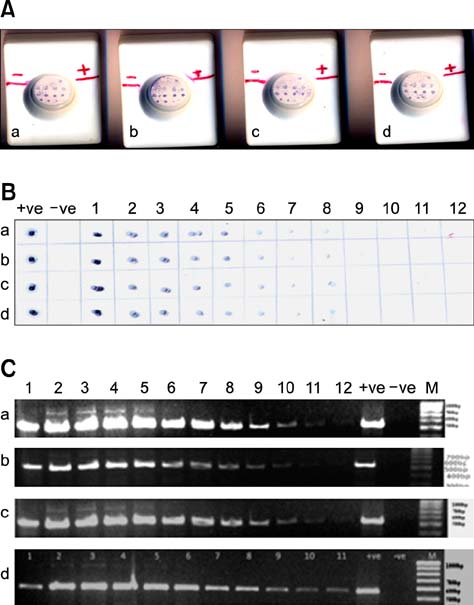
- A monoclonal antibody-based flow-through immunoassay (FTA) was developed using a nitrocellulose membrane placed on the top of adsorbent pads enclosed in a plastic cassette with a test zone at the center. The FTA could be completed within 10 min. Clear purple dots against a white background indicated the presence of Aphanomyces (A.) invadans. The FTA limit of detection was 7 microg/mL for A. invadans compared to 56 microg/mL for the immunodot. FTA and polymerase chain reaction (PCR) could detect A. invadans in fish tissue homogenates at a 10-11 dilution compared to a 10(-8) dilution by immunodot. In fish suffering from natural cases of epizootic ulcerative syndrome (EUS) collected from Mangalore, India, FTA and PCR could detect A. invadans in 100% of the samples compared to 89.04% detected by immunodot. FTA reagents were stable and produced expected results for 4 months when stored at 4~8degrees C. This rapid test could serve as simple and cost-effective on-site screening tool to detect A. invadans in fish from EUS outbreak areas and in ports during the shipment of live or frozen fish.
MeSH Terms
-
Animals
Antibodies, Monoclonal/metabolism
Aphanomyces/*isolation & purification
Fish Diseases/*diagnosis/parasitology
Fishes
Immunoassay/*methods/veterinary
India
Infection/diagnosis/parasitology/*veterinary
Polymerase Chain Reaction/methods/veterinary
Reagent Kits, Diagnostic/*standards/veterinary
Antibodies, Monoclonal
Reagent Kits, Diagnostic
Figure
Reference
-
1. Ajithkumar CR, Biju CR, Thomas R, Azeez PA. On the fishes of Puyankutty river, Kerala, India. Zoos Print J. 2001; 16:467–469.
Article2. Anil TM, Shankar KM, Mohan CV. Monoclonal antibodies developed for sensitivie detection and comparision of white spot syndrome virus isolates in India. Dis Aquat Organ. 2002; 51:67–75.
Article3. Boys CA, Rowland SJ, Gabor M, Gabor L, Marsh IB, Hum S, Callinan RB. Emergence of epizootic ulcerative syndrome in native fish of the Murray-Darling river system, Australia: hosts, distribution and possible vectors. PLoS One. 2012; 7:e35568.
Article4. Chinabut S, Roberts RJ, Willoughby GR, Pearson MD. Histopathology of snakehead, Channa striatus (Bloch), experimentally infected with the specific Aphanomyces fungus associated with epizootic ulcerative syndrome (EUS) at different temperatures. J Fish Dis. 1995; 18:41–47.
Article5. Dahal SP, Shrestha MK, Pradhan SK, Jha DK. In : Bondad-Reantaso MG, Mohan CV, Crumlish M, Subasinghe RP, editors. Occurrence of epizootic ulcerative syndrome in pond fish of Kapilvastu district of Nepal. Proceedings of the Sixth Symposium on Diseases in Asian Aquaculture; Manila: Fish Health Section, Asian Fisheries Society;2008. p. 169–178.6. Fraser GC, Callinan RB, Calder LM. Aphanomyces species associated with red spot disease: an ulcerative disease of estuarine fish from eastern Australia. J Fish Dis. 1992; 15:173–181.
Article7. Ganapathi Naik M, Rajesh KM, Sahoo AK, Shankar KM. In : Bondad-Reantaso MG, Mohan CV, Crumlish M, Subasinghe RP, editors. Monoclonal Antibody-based detection of Aphanomyces invadans for surveillance and prediction of epizootic ulcerative syndrome (EUS) outbreak in fish. Proceedings of the Sixth Symposium on Diseases in Asian Aquaculture; Manila: Fish Health Section, Asian Fisheries Society;2008. –157. –168.8. Gayathri D, Shankar KM, Mohan CV. Monoclonal antibody-based immunodot test for epizootic ulcerative syndrome pathogen, Aphanomyces invadans. Curr Sci. 2004; 87:289–291.9. Gayathri D, Shankar KM, Mohan CV, Devaraja TN. Determination of specific sampling site on EUS affected fish for diagnosis of Aphanomyces invadans by immunodot test using monoclonal antibodies. Res J Agric Biol Sci. 2008; 4:757–760.10. Johnson RA, Zabrecky J, Kiryu Y, Shield JD. Infection experiments with Aphanomyces invadans in four species of estuarine fish. J Fish Dis. 2004; 27:287–295.
Article11. Lilley JH, Callinan RB, Chinabut S, Kanchanakhan S, MacRae IH, Phillips MJ. Epizootic Ulcerative Syndrome (EUS) Technical Handbook. Bangkok: Aquatic Animal Health Research Institute;1998.12. Lilley JH, Phillips MJ, Tonguthai K. A review of Epizootic Ulcerative Syndrome (EUS) in Asia. Bangkok: Aquatic Animal Health Research Intsitute;1992.13. Lilley JH, Thompson KD, Adams A. Characterization of Aphanomyces invadans by electrophoretic and Western blot analysis. Dis Aquat Organ. 1997; 30:187–197.
Article14. Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article15. McClure FD. Design and analysis of qualitative collaborative studies: minimum collaborative program. J Assoc Off Anal Chem. 1990; 73:953–960.
Article16. Miles DJ, Thompson KD, Lilley JH, Adams A. Immunofluorescence of the epizootic ulcerative syndrome pathogen, Aphanomyces invadans, using monoclonal antibody. Dis Aquat Organ. 2003; 55:77–84.
Article17. Mohan CV, Shankar KM. Epidemiological analysis of epizootic ulcerative syndrome of fresh and brackishwater fishes of Karnataka, India. Cur Sci. 1994; 66:656–658.18. Muniruzzaman M, Chowdhury MBR. Ulcer diseases in cultured fish in Mymensingh and surrounding districts. Bangl Vet. 2008; 25:40–49.
Article19. Nsonga A, Mfitilodze W, Samui K. In : Adipala E, Tusiime G, Majaliwa JGM, editors. Epidemiology of epizootic ulceration syndrome on fish of the Zambezi river basin: a case study for Zambia. Proceedings of the Second RUFORUM Biennial Meeting; 20-24 September 2010; Entebbe. 2010. p. 1315–1318.20. Oidtmann B, Steinbauer P, Geiger S, Hoffmann RW. Experimental infection and detection of Aphanomyces invadans in European catfish, rainbow trout and European eel. Dis Aquat Organ. 2008; 82:195–207.
Article21. Phillips MJ, Keddie HG. Regional Research Programme on Relationships between Epizootic Ulcerative Syndrome in Fish and the Environment. In : A Report on the Second Technical Workshop; 13-26 August 1990; Bangkok: Network of Aquaculture Centres in Asia and the Pacific;1990. p. 133.22. Patil R, Shankar KM, Kumar BTN, Kulkarni A, Patil P, Moger N. Development of a monoclonal antibody-based flow-through immunoassay (FTA) for the detection of white spot syndrome virus (WSSV) in black tiger shrimp Penaeus monodon. J Fish Dis. 2013; 36:753–762.
Article23. Roberts RJ, Campbell B, MacRae IH. In : Proceedings of the regional seminar on epizootic ulcerative syndrome (EUS); 25-27 January 1994; Bangkok: The Aquatic Animal Health Research Institute;1994. p. 282.24. Roberts RJ, Willoughby LG, Chinabut S. Mycotic aspects of epizootic ulcerative syndrome (EUS) of Asian fishes. J Fish Dis. 1993; 16:169–183.
Article25. Saylor RK, Miller DL, Vandersea MW, Bevelhimer MS, Schofield PJ, Bennett WA. Epizootic ulcerative syndrome caused by Aphanomyces invadans in captive bullseye snakehead Channa marulius collected from south Florida, USA. Dis Aquat Organ. 2010; 88:169–175.
Article26. Shankar KM, Mohan CV. Fish and shellfish health management. Mangalore: University of Agricultural Sciences;2002. p. 104.27. European Food Safety Authority. Report of the technical hearing meeting on epizootic ulcerative syndrome (EUS). EFSA J. 2011; 9:195.28. Thompson KD, Lilley JH, Chen SC, Adams A, Richards RH. The immune response of rainbow trout (Oncorhynchus mykiss) against Aphanomyces invadans. Fish Shellfish Immunol. 1999; 9:195–210.
Article29. Vandersea MW, Litaker RW, Yonnish B, Sosa E, Landsberg JH, Pullinger C, Moon-Butzin P, Green J, Morris JA, Kator H, Noga EJ, Tester PA. Molecular assays for detecting Aphanomyces invadansin ulcerative mycotic fish lesions. Appl Environ Microbiol. 2006; 72:1551–1557.
Article30. Virgona JL. Environmental factors influencing the prevalence of a cutaneous ulcerative disease (red spot) in the sea mullet Mugil cephalus L., in the Clarence river, New South Wales, Australia. J Fish Dis. 1992; 15:363–378.
Article31. Vishwanath TS, Mohan CV, Shankar KM. Epizootic ulcerative syndrome (EUS), associated with a fungal pathogen, in Indian fishes: histopathology-'a cause for invasiveness'. Aquaculture. 1998; 165:1–9.
Article32. Zhao X, He X, Li W, Liu Y, Yang L, Wang J. Development and evaluation of colloidal gold immunochromatographic strip for detection of Escherichia coli O157. Afr J Microbiol Res. 2010; 4:663–670.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Lateral Flow Immunoassay for Antigen Detection in Human Angiostrongylus cantonensis Infection
- Quantum dot-based lateral flow immunoassay for the rapid detection of pathogens
- The Study of The Immunoassay by the Monoclonal Antibody: Flow Cytometry for Cell Surface Phenotyping
- Development of immunoassays for the detection of kanamycin in veterinary fields
- A Preliminary Study on the Development of a Fluorescence Immunochromatographic Assay for the Rapid Quantification of the Thyroid Stimulating Hormone in Serum Sample