J Bacteriol Virol.
2007 Sep;37(3):161-167. 10.4167/jbv.2007.37.3.161.
Neutralizing Antibody Induction and Cytotoxic T Lymphocyte Response to Nakayama-NIH and Beijing-1 as Japanese Encephalitis Virus Vaccine Strains
- Affiliations
-
- 1Department of Biotechnology, The Catholic University of Korea, Bucheon, 420-743, Republic of Korea. jhnam@catholic.ac.kr
- 2Department of Animal Biotechnology, College of Animal Bioscience & Technology, Konkuk University, Seoul, 143-701, Republic of Korea.
- 33Korea Center for Disease Control & Prevention, National Institute of Health, Seoul, 122-701, Republic of Korea.
- 44Biological Diagnostic Products Team Biologics Headquarters, Korea Food and Drug Administration, Seoul, 122-704, Republic of Korea.
- KMID: 1513571
- DOI: http://doi.org/10.4167/jbv.2007.37.3.161
Abstract
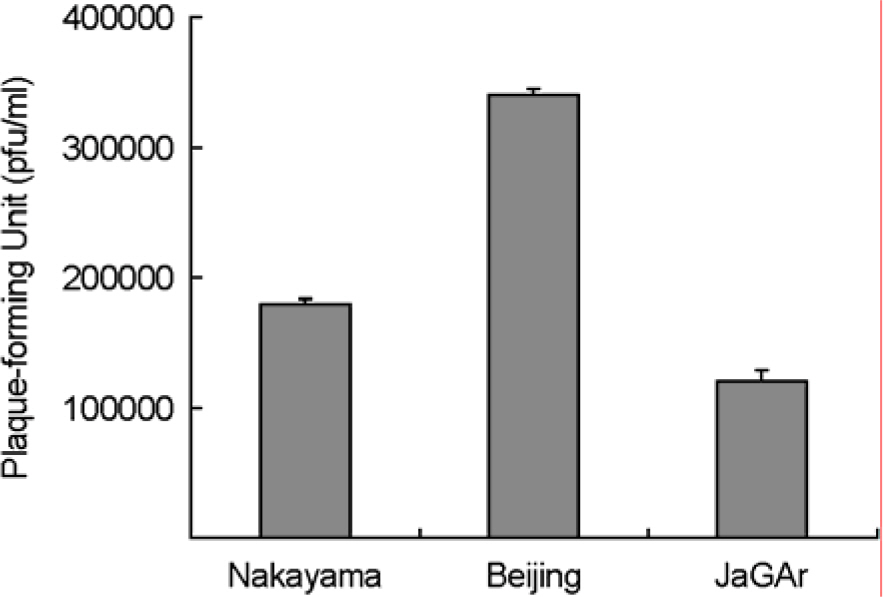
- The Japanese encephalitis virus (JEV), a member of the Flaviviridae family and Flavivirus genus, is transmitted by mosquitoes. JEV, of which some 35,000 cases are recorded every year, is a positive RNA virus. Two types of JEV vaccines have been developed to prevent the onset of encephalitis in humans, namely formalin-inactivated and liveattenuated vaccines. JEV inactivated vaccines are usually made using the Nakayama-NIH or Beijing-1 strains of the JEV virus. In this study, the immunological response to the Nakayama-NIH and Beijing-1 strains was analyzed as part of the effort to compile basic data which could lead to the selection of a suitable vaccine strain. To this end, the virus titer of Beijing-1 was found to be two-fold higher than that of Nakayama-NIH by plaque assay. Moreover, Beijing-1-induced neutralizing antibodies showed a higher level of titers when confronted by Korean JEV isolates than Nakayama-NIH-induced neutralizing antibodies (1:320 vs. 1:160, respectively). However, as a minimum ratio of 1:10 neutralizing antibody titers are required to protect against JEV infection, both strains in effect exhibited a sufficient level of neutralizing antibody titers. What's more, Beijing-1 was found to induce a somewhat higher cytotoxic T lymphocyte (CTL) response than Nakayama-NIH. Taken together, this can be taken to mean that Beijing-1 may in fact be a more effective vaccine candidate strain when it comes to inducing a high level of protective immunity against JEV infection.
MeSH Terms
Figure
Reference
-
References
1). 보건사회부. 86 급성전염병 통계연보. 1:401. 1986.2). 조해월, 김정림, 반상자, 정연준, 남재환, 이유진, 김은 정, 원은하, 최승은. 일본뇌염 백신 접종후 항 일본뇌 염 항체의 생성율과 지속기간 확인 및 신경계 부반응 의 원인규명. 국립보건원보. 32:151–158. 1995.3). 조해월, 남재환, 이유진, 김은정, 이동호, 윤경식, 고현 철. 일본뇌염바이러스 Nakayama-NIH주와 국내에서 분 리된 일본뇌염바이러스주의 유전적 차이 및 항원성 차 이의 조사. J Korean Soc Virology. 26:191–204. 1996.4). 조해월, 남재환, 이호동, 고현철, 김은정, 채수림, 이연 승. 한국에서 매개모기 (Culex tritaeniorhynchus)로부터 분리된 일본뇌염바이러스 Envelope protein의 발현과 특성분석. 국립보건원보. 33:151–169. 1996.5). Chung TJ, Nam JH, Ban SJ, Cho HW. Antigenic and Genetic analysis of Japanese encephalitis viruses isolated from Korea. Am J Trop Med Hyg. 55:91–97. 1996.6). Igarashi A. Epidemiology and control of Japaneses encephalitis. World Health Stat Q. 45:299–305. 1992.7). Kitano T, Yabe S, Kobayashi M, Oya A, Ogata T. Immunogenicity of JE Nakayama and Beijing-1 vaccines. JE & HFRS Bull. 1:37–41. 1986.8). Ku CC, King CC, Lin CY, Hsu HC, Chen LY, Yueh YY. Homologous and heterologous neutralization antibody responses after immunization with Japanese encephalitis vaccine among Taiwan children. J Med Virol. 44:122–131. 1994.
Article9). Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine. 18:33–35. 2000.
Article10). Nam JH, Chae SL, Park SH, Jeong YS, Joo MS, Kang CY, Cho HW. High level of sequence variation in the 3′ non-coding region of Japanese encephalitis viruses isolated in Korea. Virus Genes. 24:21–27. 2002.11). Nam JH, Chae SL, Won SY, Kim EJ, Yoon KS, Kim BI, Jeong YS, Cho HW. Short report: genetic heterogeneity of Japanese encephalitis virus assessed via analysis of the full-length genome sequence of a Korean isolate. Am J Trop Med Hyg. 65:388–392. 2001.
Article12). Nam JH, Chung YJ, Ban SJ, Kim EJ, Park YK, Cho HW. Envelope gene sequence variation among Japanese encephalitis viruses isolated in Korea. Acta Virol. 40:303–309. 1996.13). Nimmannitya S, Hutamai S, Kalayanarooj S, Rojanasuphot S. A field study on Nakayama and Beijing strains of Japanese encephalitis vaccines. Southeast Asian J Trop Med Public Health. 26:689–693. 1995.14). Okuno T, Okada T, Kondo A, Suzuki M, Kobayashi M, Oya A. Immunotyping of different strains of Japanese encephalitis virus by antibody-absorption, haemagglutination-inhibition and complement-fixation tests. Bull World Health Organ. 38:547–563. 1968.15). Oya A. new development of criteria on Japanese encephalitis vaccine requirements in Japan. JE HFRS Bull. 2:11–13. 1987.16). Pan CH, Chen HW, Huang HW, Tao MH. Protective mechanisms induced by a Japanese encephalitis virus DNA vaccine: requirement for antibody but not CD8(+) cytotoxic T-cell responses. J Virol. 75:11457–11463. 2001.17). Plesner AM. Allergic reactions to Japanese encephalitis vaccine. Immunol Allergy Clin North Am. 23:665–697. 2003.
Article18). Sabin AB, Schledinger RW, Ginder PR, Matsumoto M. Japanese encephalitis American soldiers in Korea. Am J Hyg. 46:356–375. 1947.19). Shyu WRH, Wang YC, Chin C, Chen WJ. Assessment of neutralizing antibodies elicited by a vaccine (Nakayama) strain of Japanese encephalitis virus in Taiwan. Epidemiol Infect. 119:78–83. 1997.
Article20). Sumiyoshi H, Mori C, Fuke I, Morita K, Kuhara S, Kondou J, Kilkuchi Y, Nagamutu H, Igarashi A. Complete nucleotide sequence of the Japaneses encephalitis virus genome RNA. Virology. 161:497–510. 1987.21). Tsai TF, Yu YX. Japanese encephalitis vaccines. In. plotkin SA, Mortimer EA, editors. Vaccines WA Saunders;p. 671–714. 1994.22). Wu CJ, Huang HW, Tao MH. Induction of cross-protection against two wild-type Taiwanese isolates of Japanese encephalitis virus using Beijing-1 strain DNA vaccine. Vaccine. 21:3938–3945. 2003.
Article23). Yun SI, Kim SY, Choi WY, Nam JH, Ju YR, Park KY, Cho HW, Lee YM. Molecular characterization of the full-length genome of the Japanese encephalitis viral strain K87P39. Virus Res. 96:129–140. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of the Three Dimensional Structure of Envelope Protein of the Japnes Encephalitis virus Isolated in Korea
- Studies on the Duration of Immunity and Production of Antibody following Immunization with Inactivated Killed Japanese Encephalitis Vaccine
- Development of Neutralization Assay using Murine Leukemia Virus (MuLV) Pseudotyped with Japanese encephalitis Virus (JEV) env Gene
- Immune Response to SA14-14-2 Live Attenuated Japanese Encephalitis Vaccine
- Comparison of the antigenic relationship between Japanese encephalitis virus genotypes 1 and 3