J Bacteriol Virol.
2007 Mar;37(1):47-59. 10.4167/jbv.2007.37.1.47.
Study on Detection, Isolation and Identifications of Infectious Viruses in Various Water Samples
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Kangwon National University, Chonchun, Kangwon-do 200-701, Korea. yungoh@kangwon.ac.kr
- 2Department of Microbiology, College of Medicine, Koshin University, Yungdo-Ku, Pusan, 606-701, Korea.
- KMID: 1513552
- DOI: http://doi.org/10.4167/jbv.2007.37.1.47
Abstract
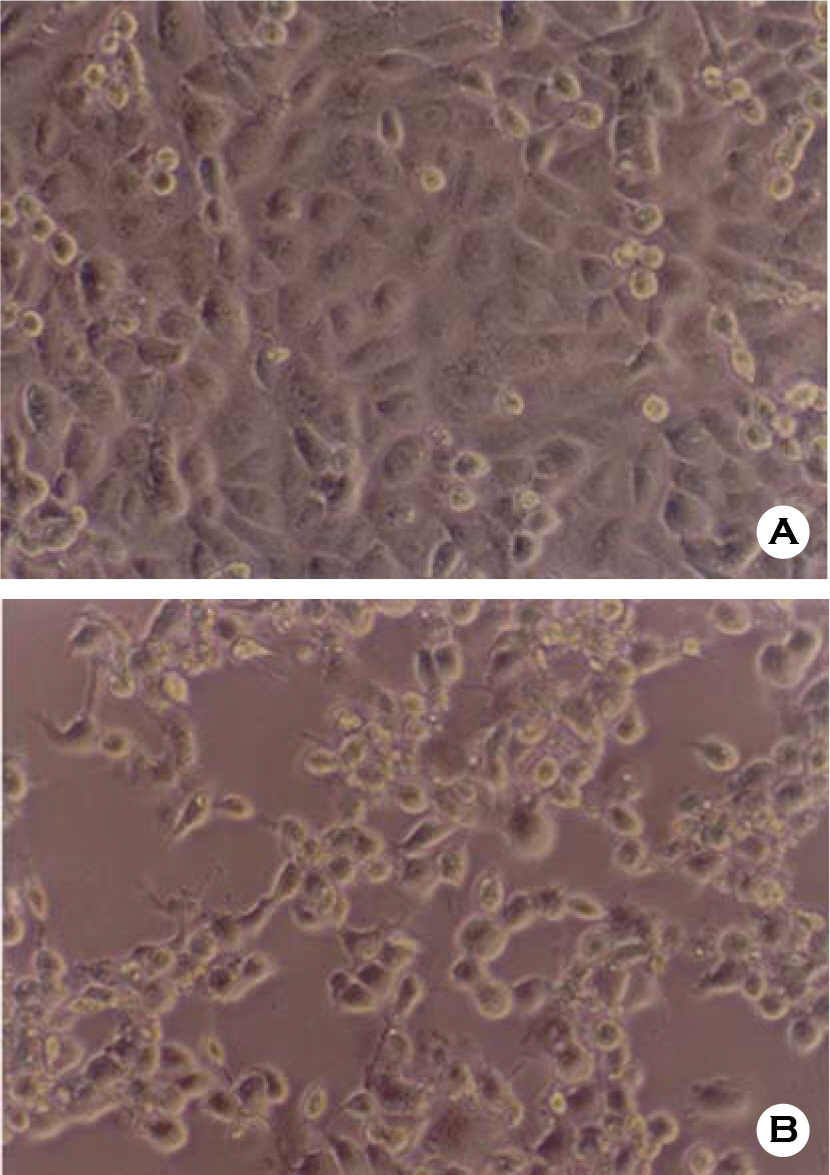
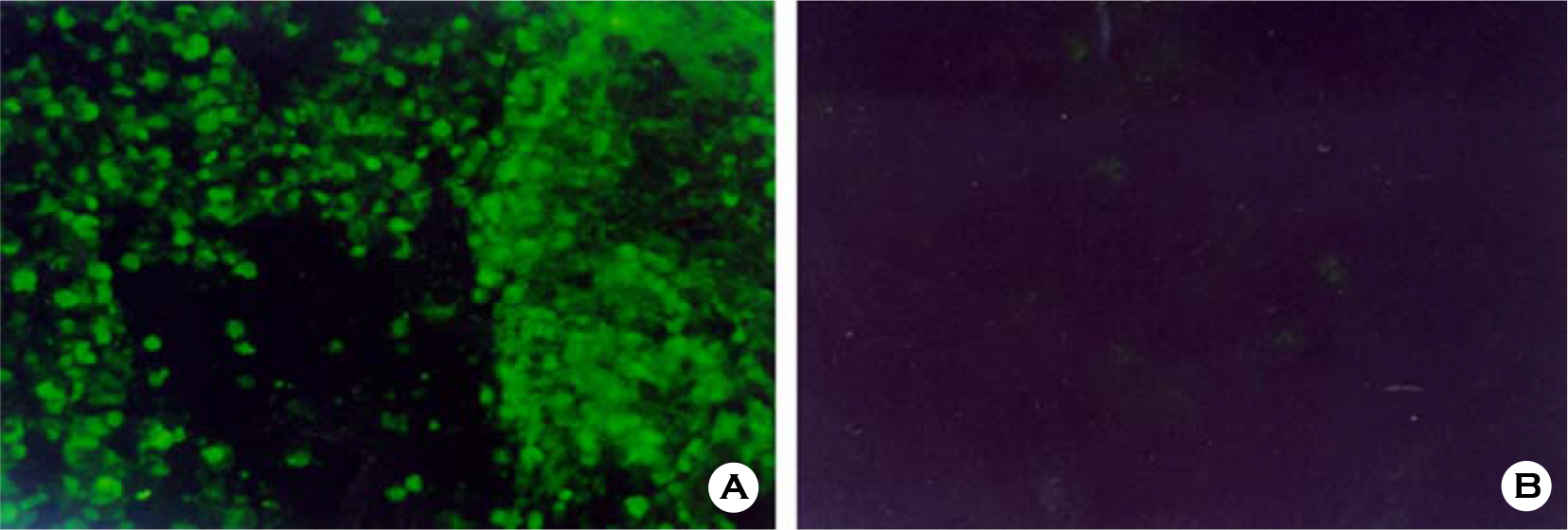
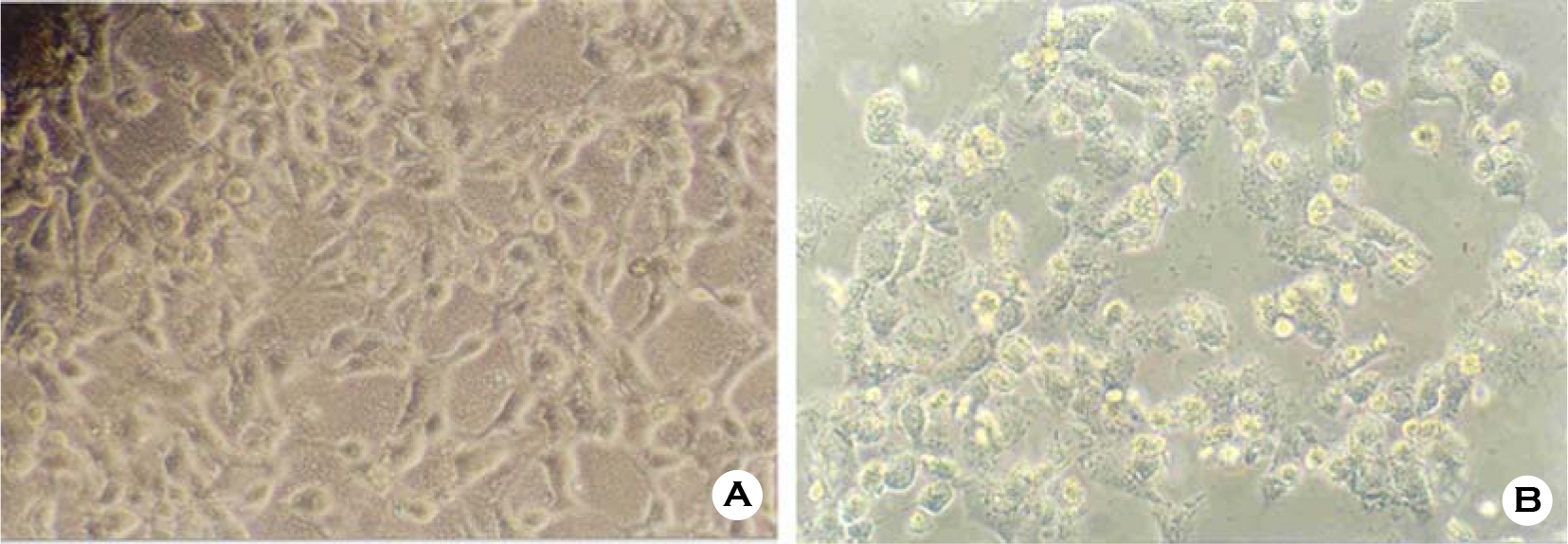
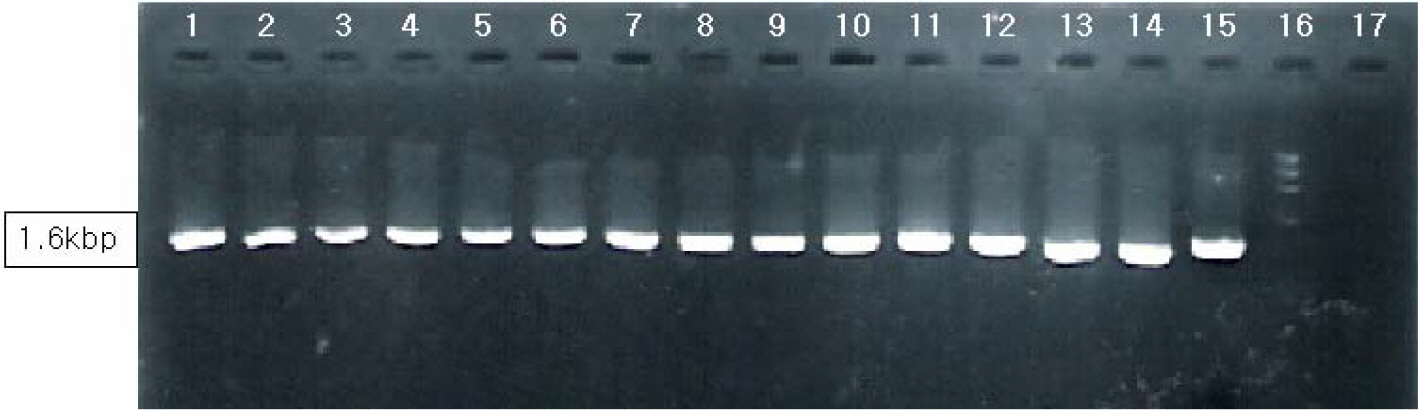
- Various factors using cell lines can effect kinds and frequencies of infectious viruses obtained in the detection tests on various water samples. We tried to find out technical problems for the maximum virus isolations from water samples and characterize the virus isolates from waters in nature and in various purification stages. Fourteen viruses were isolated from 169 water samples by virus monitoring protocol for the information collection requirements rule, US EPA. The morphological changes caused by viruses and mycoplasma infections were compared with for increasing the specificity of tests employed. Cytopathic effects of slow growing viruses were found very similar with those by toxic effects in water samples and mycoplasma infections. Five of 6 stream water samples tested (83.33%) showed virus contaminations with the range of 1.03 to 5.75 MPNs/100 liter. Eight of 24 source water samples (33.35%) showed viral contaminations. One water sample of 24 water samples during precipitation stages was shown to include infectious viruses. It was confirmed that infectious viruses were significantly decreased by purification stages from streams. The titers (TCID50) of virus isolates were ranged as 10(-6.8) ~ 10(-6.925)/ml. The virus isolates were identified by immune fluorescent antibody (IFA) method using virus specific immune sera and serotyped using serotype specific reference sera. Of 14 virus isolates, 7 samples were identified as poliovirus and the other 7 were identified as coxsakie virus. Of 7 polioviruses, one was serotyped as type I, 3 viruses as type II and another 3 as type III. Conclusively, BGM cell lines must be free of mycoplasma for the strict examination of infectious viruses in water and highly sensitive for mainly enteroviruses. In addition, most of infectious viruses showing typical cytopathic effect from water samples were confirmed as coxsackie B and live attenuated vaccine strains of 3 polio types when BGM cells were used for virus isolations.
MeSH Terms
Figure
Reference
-
References
1). 日本上水道協會. 上水試驗方法2001年版-追補版. 222:1–222. 2006.2). Alberto CA, Barbara SC, Edith CS, Peter FW. Diagnostic tests for poliovirus infection: a comparison of neutralization and immunofluorescence for the identification and typing of stool isolates. Journal of Virological Methods. 52(1–2):35–39. 1995.
Article3). Anne VN, Richard D, Gerard C, Freddy H. Interaction between turkey monocytes and avian Chlamydia psittaci in the presence of Mycoplasma sp.: the importance of nitric oxide. Developmental & Comparative Immunology. 24(4):417–432. 2000.4). APHA-AWWA-AEF. Detection of enteric viruses, Standard methods for the examination of water and waste water, 19th edition. 1998.5). Australia National Health and Medical Research Council. Australia Drinking Water Guidelines. 2004.6). Bern C, Martines J, DeZoyasa I, Glasses RI. The magnititute of the global problem of diarrhocal disease: A ten-year update,: Bull. World Health Organ. 70:705–714. 1992.7). Berg G, Dahling DR, Berman D. Recovery of small quantities of viruses from clean waters on cellulose nitrate membrane filters. Appl Microbiol. 22(4):608–614. 1971.
Article8). Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 2:1281–1283. 1970.
Article9). Craun GF, McCabe LJ. Review of causes of waterborne diseases outbreaks. J Amer Water Works Ass. 65:74–84. 1973.10). Cubitt WD. Caliciviruses. In. Kapikian AZ, editor. Virus Infections of the Gastrointestinal Tract. New York: Marcel Dekker;p. 549–568. 1994.11). Cubitt WD, Holzel H. An outbreak of rotavirus infection in a long-stay ward of a geriatric hospital. J Clin Patholo. 33:306–308. 1980.
Article12). Dahling DR, Wright BA. Processing and transport of environ mental virus samples. Appl Environ Microbiol. 47(6):1272–1276. 1984.13). Dahling DR, Wright BA. Recovery of viruses from water by a modified flocculation procedure for second-step concentration. Appl Environ Microbiol. 51(6):1326–1331. 1986.
Article14). Dolin R, Blacklow NR, DuPont H, Buscho RF, Wyatt RG, Kasel JA, Hornick R, Chanock RM. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med. 140:178–583. 1972.
Article15). Enrquez CE, Abbaszadegan M, Pepper IL, Richardson KJ, Gerba CP. Poliovirus detection in water by cell culture and nucleic acid hybridization. Wat Res. 27(7):1113–1118. 1993.16). Enrquez CE, Gerba CP. Concentration of enteric adenovirus 40 from tap, sea waste water. Wat Res. 29(11):2554–2560. 1995.17). Erwin DB, Erwin D, Harry V, Marion PGK. Diagnosis of Norovirus outbreaks by commercial ELISA or RT-PCR. Journal of Virological Methods. 137(2):259–264. 2006.
Article18). Fox JP, Brandt CD. Wassermann FE: The Virus Watch Program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, effciency of surveillance, patterns of infection and relation to illness. Am J Epidemiol. 89:25–50. 1969.19). Gardner PS, McQuillin J. Rapid Virus Diagnosis Application of immunofluorescence 1st ed. Butterworths. 1974.20). Green KY, Chanock RM. Kapikian: Human calicivirus, in Fields. Virology. 841–867:2001.21). Hill WF Jr, Jakubowski W, Akin EW, Clarke NA. Detection of virus in water: sensitivity of the tentative standard method for drinking water. Appl Environ Microbiol. 31(2):254–261. 1976.
Article22). Jackson GG, Muldoon RL, Cooper RS. Reovirus type 1 as an etiologic agent of the common cold. J Clin Invest. 40:1051. 1961.23). John P. Cell and Tissue Culture 5th ed. Churchill Livingstone. 1975.24). Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Chanock RM, Kalica AR. Visualilzation by immune electron microscopy of a 27-nm particle associated with acute infectious non-bacterial gastroenteritis. 10:1. J Virol. 10:1075–1081. 1972.25). Kapikian AZ, Yolken RH, Greenberg HB. Gastroenteritis viruses. In:. Lennette EH, Schmidt NJ, editors. eds,. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. 5th ed.Washington, DC: American Public Health Association;p. 927–995. 1979.26). Lennette EH, Schmidt NS. Diagnostic procedures for viral, rickettsial and chlamydial infection (5th edition). American Public Health Association. 1979.27). Li JW. A new and simple method for concentration of enteric viruses from water. J Virol Methods. 74(1):99–1081. 1998.28). Logan KB, Scott GE, Seeley ND, Primrose SB. A portable device for the rapid concentration of viruses from large volumes of natural freshwater. J Virol Methods. 3(4):241–249. 1981.
Article29). Lund E. Disposal of Sludges, in Virus in Water, Interdisciplinary Books. Washington D.C.1976.30). Madley CR, Cosgrove BP. 28 nm in infantile gastroenteritis. Lancet. 451–452:1975.31). Melnick JL, Dow RP. Poliomyelitis in Hidalgo County, Texas, 1948. Poliomyelitis and coxsackie viruses from fllies. Am J Hyg. 58:288–309. 1953.32). Nestor I. Investigations on the presence of enteroviruss in drinking water. Viology. 29(3):203–207. 1978.33). Pallin R, Wyn-Jones AP, Place BM, Lightfoot NF. The detection of enteroviruses in large volume concentrates of recreational waters by the polymerase chain reaction. J Virol Methods. 67(1):57–67. 1997.
Article34). Pertschuk LP, Broome JD, Brigati DJ, Cook AW, Vuletin JC, Rainford EA, Gupta JK, Kim DS, Nidsgorski F. Jejunal immunopathology in amyotrophic lateral sclerosis and multiple sclerosis identification of viral intigens by immunofluore-scence. The Lancet. 309(8022):1119–1123. 1977.35). Provost PJ, Hilleman MR. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 160:213–221. 1979.
Article36). Payment P. Evaluation of the efficiency of the adsorption elution technic for poliovirus 1 on fiber glass filters: application in the virological analysis of 100 ml to 1000 ml of water. Can J Microbiol. 24(11):1413–1416. 1978.37). Price WH. The isolation of a new virus associated with respiratory cllinical disease in humans. Proc Natl Acad Sci USA. 42:892–896. 1956.38). Quignon F, Kiene L, Levi Y, Sardin M, Schwartzbrod L. Virus behaviour within a distribution system. Wat Sci Tech. 35(11–12):311–318. 1997.
Article39). Quignon F, Senwartzbrod L. Influence of salts and montmorillonite upon heat inactivation of poliovirus in sterile water. Wat Sci Technol. 31(5–6):177–180. 1995.
Article40). Ramia S. Second-step concentration of viruses in drinking and surface waters using polyethylene glycol hydroextraction. Can J Microbiol. 25(5):587–592. 1979.
Article41). Richards AF, Lopman B, Gunn A, Curry A, Ellis D, Cotterill H, Ratcliffe S, Jenkins M, Appleton H, Gallimore CI. Evaluation of a commercial ELISA for detecting Norwalklike virus antigen in faeces. Journal of Clinical Virology. 26(1):109–115. 2003.
Article42). Robert LA, Mary KE. The Epidemiologic and Clinical Importance of Norovirus Infection. Gastroenterology Clinics of North America. 35(2):275–290. 2006.
Article43). Rosemary CS, Gwen C, Erick B, Janine L, Cathy AP. Comparison of multiple shell vial cell lines for isolation of enteroviruses: A national perspective. Journal of Clinical Virology. 37(3):151–155. 2006.
Article44). Rosen L. Reoviruses, Diagnostic Procedures for viral, rickettsial and chlamydial infections, Edwin H. Lennette. 5th edith.577–584. 1980.45). Sato K, Inaba Y, Shinozaki T. Isolation of human rotavirus in cell cultures. Arch Virol. 69:155–160. 1981.
Article46). Schaffer FL, Bachrach HL, Brown F. Caliciviridae. Intervirology. 14–16:1980.
Article47). Shaub SA, Sorber CA. Viruses on Solids in water, in Virus in Water, Interdisciplinary Books. Washington D.C.1976.48). Schmidt N. cell culture techniques for diagnostic virology, Diagnostic Procedures for viral, rickettsial and chlamydial infections, Edwin H. Lennette. 5th edith.116–120. 1980.49). Tam AW, White R, Yarbough PO, Murphy BJ, McAtee CP, Lanford RE, Fuerst TR. In vitro infection and replication of hepatitis E virus in primary cynomolgus macaque hepatocytes. Virology. 238:94–102. 1997.50). Taylor JW, Gary GW Jr, Greenberg HB. Norwalk-related viral gastroenteritis due to contaminated drinking water. Am J Epidemiol. 114(4):584–592. 1981.
Article51). Toranzos GA, Gerba CP. An improved method for the concentration of rotaviruses from large volumes of water. J Virol Methods. 24(1–2):131–140. 1989.
Article52). Tsai YL, Tran B, Sangermano LR, Palmer CJ. Detection of poliovirus, hepatitis A virus, and rotavirus from sewage and ocean water y triplex reverse transcriptase PCR. Appl. Environ Microbiol. 60(7):2400–2407. 1994.53). Tyler , Kenneth L. Mammalina reoviruses. in. Virology. Lippincott Williams & Wilkins;p. 1729–1745. 2001.54). Urasawa T, Urasawa S, Taniguchi K. Sequential passages of human rotavirus in MA 104 cells. Microbiol Immunol. 25:1025–1035. 1981.55). US EPA. The US EPA Maual of Methods for Virology. 1984.56). US EPA. Virus monitoring protocol for the information collection requirements rule, EPA/814/B/95B002. 1995.57). Wong-Lee JG, Lovett M. Rapid and sensitive PRC method for identification of Mycoplasma species in tissue culture, in Diagnostic Molecular Microbiology Principles and Applications, Amecican Society for Microbiology, Washington, D.C. 257–265. 1993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization and Detection of Enteric Viruses in Surface Water, Finished Water, Tap Water by Total Culturable Virus Assay (TCVA) Method
- Detection of Enteroviruses and Mammalian Reoviruses by RT-PCR and Integrated Cell Culture-PCR in CPE-positive Surface Water Samples
- Study of the Detection of Enteric Viruses and Bacteria in Spring-water and Groundwater in Busan ('10~'11)
- Integrated Cell Culture-PCR Detection of Enteroviruses and Reoviruses in Water Sources in Gyeonggi-do
- Influence of Physicochemical Environmental Factors on the Occurrence of Waterborne Viruses in Korean Surface Water