J Korean Soc Radiol.
2011 Aug;65(2):161-166. 10.3348/jksr.2011.65.2.161.
Clinical Usefulness of 18F 2-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography Scan in the Diagnosis of Ampullary Carcinoma
- Affiliations
-
- 1Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jjchung@yuhs.ac
- KMID: 1443480
- DOI: http://doi.org/10.3348/jksr.2011.65.2.161
Abstract
- PURPOSE
To evaluate the clinical usefulness of the 18F 2-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) scan in the diagnosis of the ampulla of Vater cancer.
MATERIALS AND METHODS
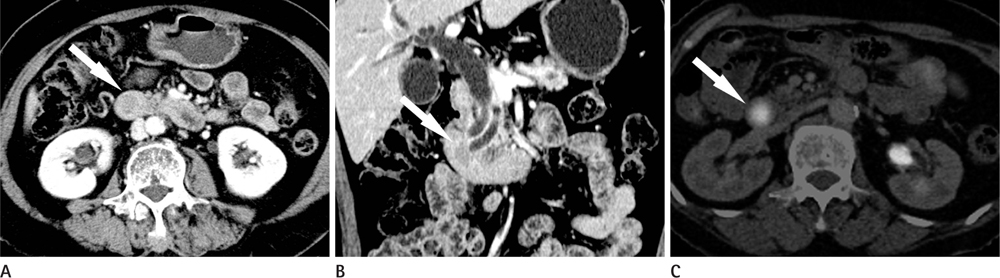
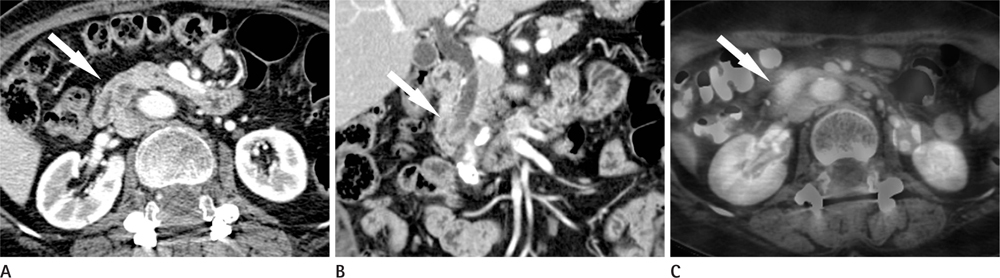
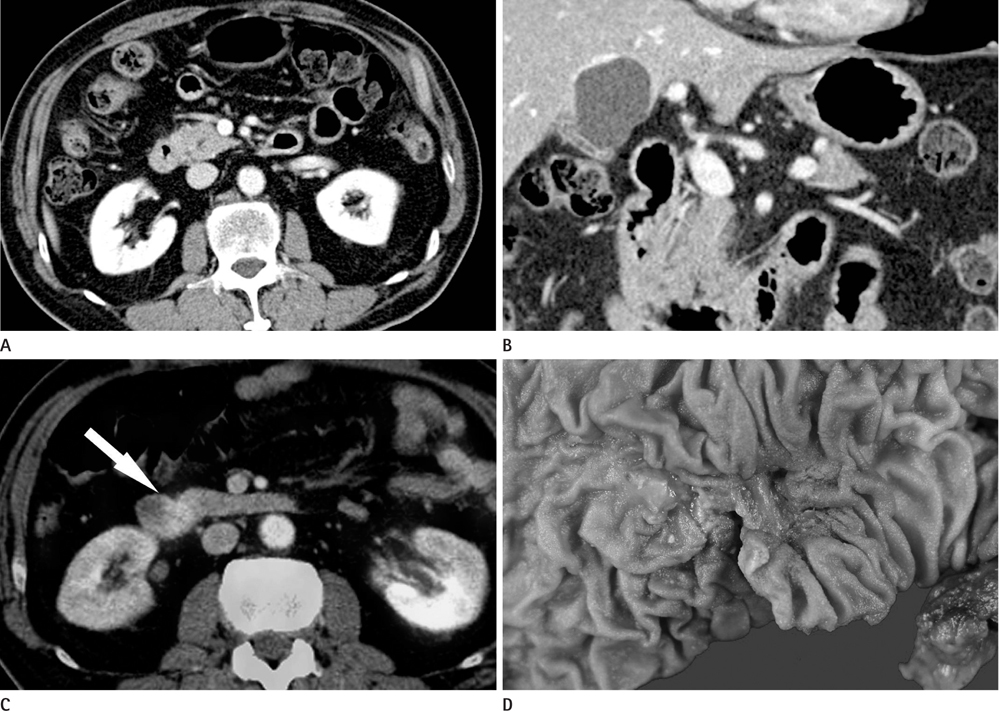
CT images of 39 patients with ampulla of Vater cancer were reviewed regarding the lesion size, location, and bile or pancreatic duct dilatation. The patients were divided into three groups according to the lesion visibility on CT (Group A: visible mass, Group B: no visible mass but prominent ampulla, Group C: no visible lesion). Standardized uptake value (SUV) was measured on PET scan and the detection rate on PET images was comparable with that of CT images.
RESULTS
Twenty-four patients (61.5%) were classified as Group A, 11 (28.2%) as Group B and 4 (10.3%) as Group C. All of Group A, 10 (90.9%) of Group B and 3 (75.0%) of Group C showed biliary dilatation. Pancreatic duct dilatation was shown in 18 (75.0%) of Group A, 9 (81.8%) of Group B, and 1 (25.0%) of Group C. The average of SUV of all patients was 5.90 +/- 3.1. Most (94.9%) of all patients showed high FDG uptake over 2.5 with 93.9% in Group B and C.
CONCLUSION
18F-FDG PET scan was use for the detection of ampulla of Vater cancer, even though the lesion was invisible on CT.
MeSH Terms
Figure
Reference
-
1. Buck JL, Elsayed AM. Ampullary tumors: radiologic-pathologic correlation. Radiographics. 1993; 13:193–212.2. Neoptolemos JP, Talbot IC, Carr-Locke DL, Shaw DE, Cockleburgh R, Hall AW, et al. Treatment and outcome in 52 consecutive cases of ampullary carcinoma. Br J Surg. 1987; 74:957–961.3. Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000; 12:75–79.4. Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009; 100:598–605.5. Goodman MT, Yamamoto J. Descriptive study of gallbladder, extrahepatic bile duct, and ampullary cancers in the United States, 1997-2002. Cancer Causes Control. 2007; 18:415–422.6. Morris-Stiff G, Alabraba E, Tan YM, Shapey I, Bhati C, Tanniere P, et al. Assessment of survival advantage in ampullary carcinoma in relation to tumour biology and morphology. Eur J Surg Oncol. 2009; 35:746–750.7. Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008; 12:1422–1428.8. Sommerville CA, Limongelli P, Pai M, Ahmad R, Stamp G, Habib NA, et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol. 2009; 100:651–656.9. Chen WX, Xie QG, Zhang WF, Zhang X, Hu TT, Xu P, et al. Multiple imaging techniques in the diagnosis of ampullary carcinoma. Hepatobiliary Pancreat Dis Int. 2008; 7:649–653.10. Edge MD, Hoteit M, Patel AP, Wang X, Baumgarten DA, Cai Q. Clinical significance of main pancreatic duct dilation on computed tomography: single and double duct dilation. World J Gastroenterol. 2007; 13:1701–1705.11. Skordilis P, Mouzas IA, Dimoulios PD, Alexandrakis G, Moschandrea J, Kouroumalis E. Is endosonography an effective method for detection and local staging of the ampullary carcinoma? A prospective study. BMC Surg. 2002; 2:1.12. Pham DT, Hura SA, Willmann JK, Nino-Murcia M, Jeffrey RB Jr. Evaluation of periampullary pathology with CT volumetric oblique coronal reformations. AJR Am J Roentgenol. 2009; 193:W202–W208.13. Kim JH, Kim MJ, Chung JJ, Lee WJ, Yoo HS, Lee JT. Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics. 2002; 22:1335–1352.14. Mahnke D, Chen YK, Antillon MR, Brown WR, Mattison R, Shah RJ. A prospective study of complications of endoscopic retrograde cholangiopancreatography and endoscopic ultrasound in an ambulatory endoscopy center. Clin Gastroenterol Hepatol. 2006; 4:924–930.15. Sperti C, Pasquali C, Fiore V, Bissoli S, Chierichetti F, Liessi G, et al. Clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the management of patients with nonpancreatic periampullary neoplasms. Am J Surg. 2006; 191:743–748.16. Hustinx R, Bénard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med. 2002; 32:35–46.17. Kalady MF, Clary BM, Clark LA, Gottfried M, Rohren EM, Coleman RE, et al. Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol. 2002; 9:799–806.18. Al-Sugair A, Coleman RE. Applications of PET in lung cancer. Semin Nucl Med. 1998; 28:303–319.19. Kim S, Lee NK, Lee JW, Kim CW, Lee SH, Kim GH, et al. CT evaluation of the bulging papilla with endoscopic correlation. Radiographics. 2007; 27:1023–1038.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 18F-2-Deoxy-2-Fluoro-D-Glucose Positron Emission Tomography: Computed Tomography for Preoperative Staging in Gastric Cancer Patients
- Positron Emission Tomography: Application in Pediatric Epilepsy
- 18F-fluoro-2-deoxy-D-glucose positron emission tomography findings of neurolymphomatosis
- Non-Malignant 18F-FDG Uptake in the Thorax by Positron Emission Tomography Computed Tomography Fusion Imaging
- Clinical Usefulness of a PET Scan in the Diagnosis of a Recurrent Colorectal Carcinoma