Lab Med Online.
2012 Jan;2(1):10-14. 10.3343/lmo.2012.2.1.2.
Evaluation of HbA1c Levels Via the Latex Immunoturbidimetric Method by Using Chemistry Autoanalyzer
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. lyejh@catholic.ac.kr
- KMID: 1435746
- DOI: http://doi.org/10.3343/lmo.2012.2.1.2
Abstract
- BACKGROUND
Measurement of HbA1c levels is widely used to diagnose diabetes mellitus and to evaluate and monitor plasma-glucose concentrations over 6-8 weeks. In this study, we evaluated the diagnostic performance of the newly developed latex immunoturbidimetric method by using Autolab HbA1c.
METHODS
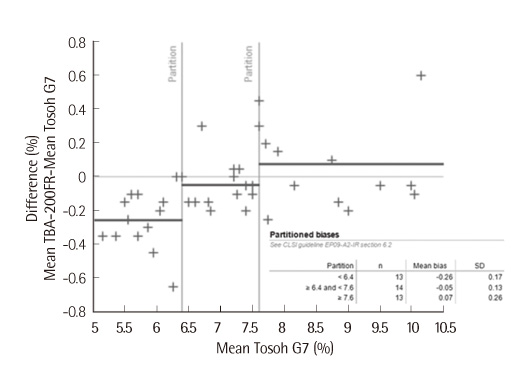
We analyzed and compared the diagnostic performance of Autolab HbA1c with that of Toshiba 200FR between April 2009 and July 2009. According to guidelines (EP5-A2, EP6-P, EP9-A2) of the clinical and laboratory standards institute (CLSI), we compared linearity, precision and correlation of Autolab HbA1c with those of G7 (Tosoh Corp., Kyoto, Japan) by using high-performance liquid chromatography (HPLC) method.
RESULTS
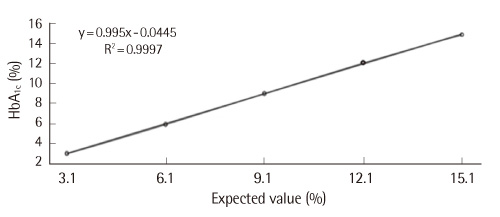
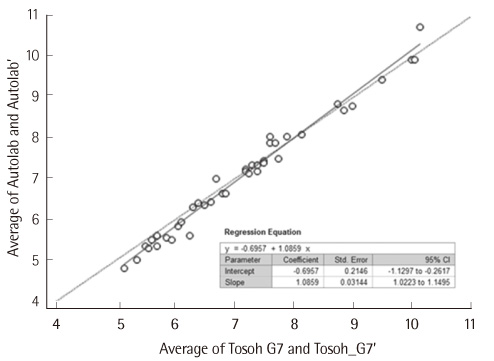
Data obtained using Autolab HbA1c showed good linearity in mixtures of samples with low (3.1%) and high (15.1%) levels of HbA1c (r2 = 0.9997). In the analysis of within-run precision of the samples with HbA1c levels of 5.1% and 12.1%, the SDs were 0.04 and 0.06 and covariances of these samples were 0.8% and 0.5%, respectively. In the Deming regression model, the regression equation was as follows: Autolab HbA1c = 1.0859xTosoh HPLC-0.6957.
CONCLUSIONS
In this study, Autolab HbA1c method showed better performance characteristics than Tosoh G7 did. In reference review, there was no interference of variant hemoglobin. The data acquisition time of Autolab HbA1c was lower than that of Tosoh G7. The advantages of Autolab HbA1c are that it can be used as an autoanlyzer in routine chemical analysis, it does not require pre-analytical treatment, and the samples are automatically treated with distilled water for hemolysis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of Analytical Performance of an Automated Glycated Hemoglobin Analyzer, HLC-723 G11
Yoo Na Chung, Seung Gyu Yun, Yunjung Cho
Lab Med Online. 2020;10(1):46-51. doi: 10.3343/lmo.2020.10.1.46.
Reference
-
1. Global burden: prevalence and projections. 2010 and 2030. International diabetes federation. Updated on Nov 2010. http://www.idf.org/diabetesatlas/diabetesand-impaired-glucose-tolerance.2. Chronic disease statistics of Korea. Statistics Korea. Updated on May 2011. http://www.index.go.kr/egams/stts/jsp/potal/stts/PO_STTS_IdxMain.jsp?idx_cd=1438.3. Cause of death statistics of Korea. Statistics Korea. Updated on Nov 2010. http://kostat.go.kr/portal/korea/kor_nw/2/6/1/index.board?bmode=read&bSeq=&aSeq=179505&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt.4. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.5. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011. 34:Suppl 1. S62–S69.7. Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 2006. 4th ed. Washington, D.C: Saunders;880–883.8. Molinaro RJ. Targeting HbA1c: standardization and clinical laboratory measurement. MLO Med Lab Obs. 2008. 40:10–14. 16–19.9. Song J, Kwon KC, Kim JH, Kim JW, Min WK, Lee SY, et al. Annual report on external quality assessment in metabolic disorders in Korea (2009). J Lab Med Qual Assur. 2010. 32:131–146.10. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. Document EP6-A. 2003. Wayne, PA: Clinical and Laboratory Standards Institute.11. Clinical and Laboratory Standards Institute. Document EP5-A2. Evaluation of precision performance of quantitative measurement methods; approved guideline. 2004. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute.12. Clinical and Laboratory Standards Institute. Document EP9-A2. Method comparison and bias estimation using patient samples; approved guideline. 2002. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute.13. Little RR, Sacks DB. HbA1c: how do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes. 2009. 16:113–118.14. Caragher TE, Dohnal JC, Lomont ME. Cautionary note regarding HbA1c methods predicting the clinical status of diabetic patients. Diabetes Care. 2000. 23:867–869.
Article15. Kilpatrick ES. HbA1c measurement. J Clin Pathol. 2004. 57:344–345.16. Hanas R, John G. International HbA1c Consensus Committee. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care. 2010. 33:1903–1904.
Article17. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009. 3:446–451.
Article18. Jones TG, Warber KD, Roberts BD. Analysis of hemoglobin A1c from dried blood spot samples with the Tina-quantR II immunoturbidimetric method. J Diabetes Sci Technol. 2010. 4:244–249.
Article19. Lee ST, Kim MS, Choi DY, Kim SK, Ki CS. Incidence of variant hemoglobin (Hb) and increased fetal Hb concentrations and their effect on HbA1c measurement in a Korean population. Clin Chem. 2006. 52:1445–1446.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the AutoLab HbA1c Reagent by Using Hitachi Clinical Analyzer 7180
- Evaluation of Bio-Rad D-10 HbA1c Autoanalyzer
- Evaluation for the Precision, Linearity and Comparision of In Wha Liqchem(R) Reagent for the Chemistry Autoanalyzer
- Evaluation of the Nanopia P-FDP Reagent Kit for Use in the STA-R EVOLUTION Automated Blood Coagulation Analyzer
- The Study of Latex Allergy in the Operating Room Nurses