Korean J Physiol Pharmacol.
2013 Feb;17(1):37-42. 10.4196/kjpp.2013.17.1.37.
Taxifolin Glycoside Blocks Human ether-a-go-go Related Gene K+ Channels
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. haena@cau.ac.kr
- 2Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 3Department of Dermatology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 4Department of Family Medicine, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 5Department of Cosmetology Science, Nambu University, Gwangju 506-706, Korea.
- 6College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 1432761
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.37
Abstract
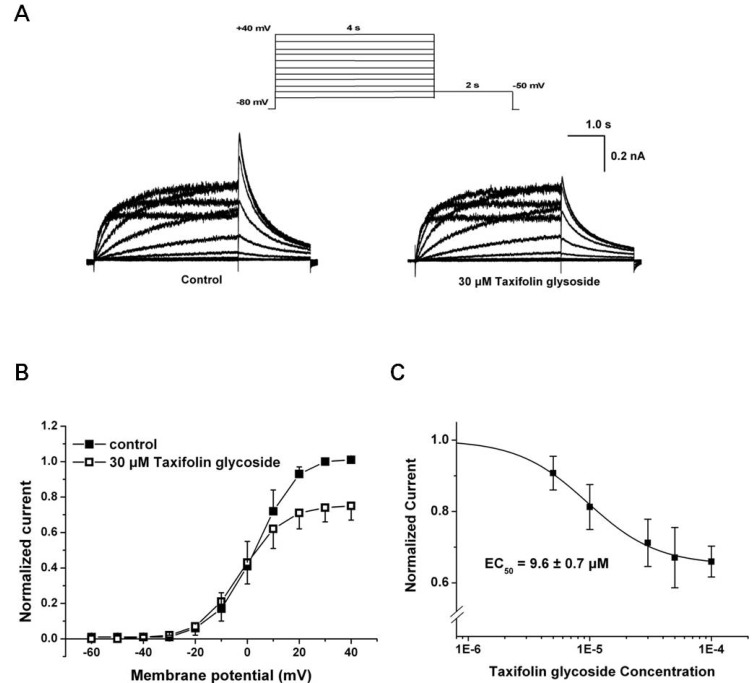
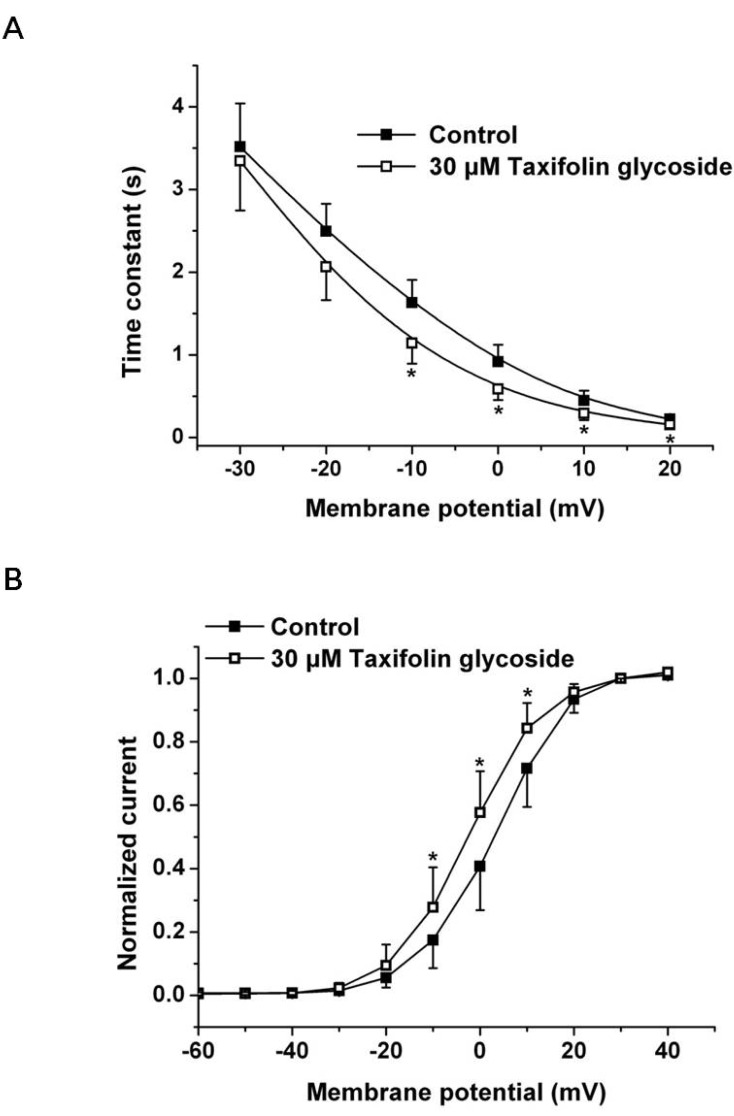
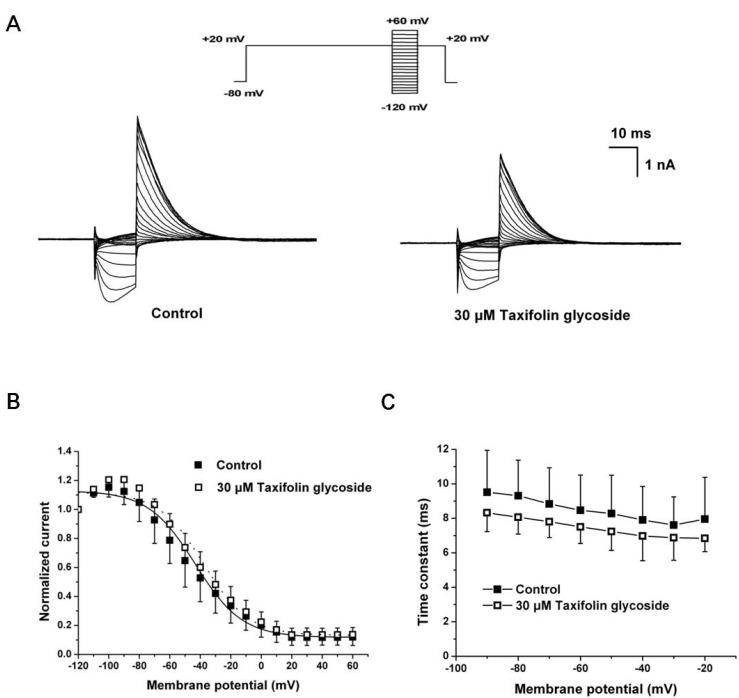
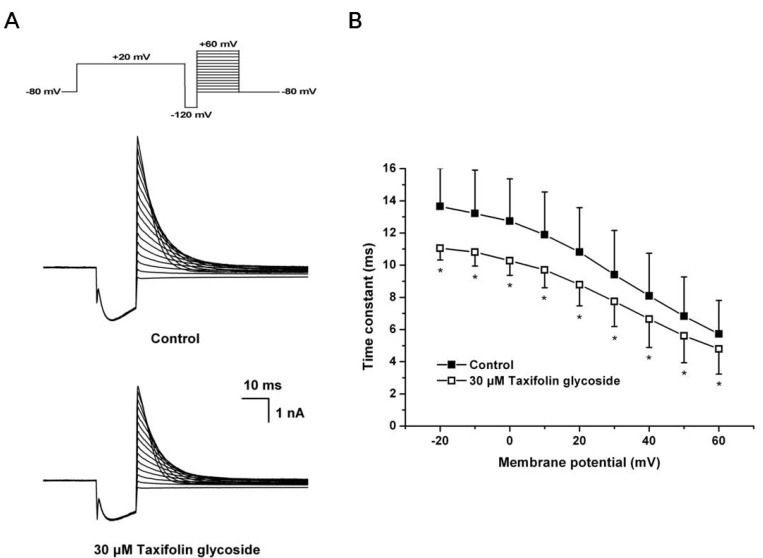
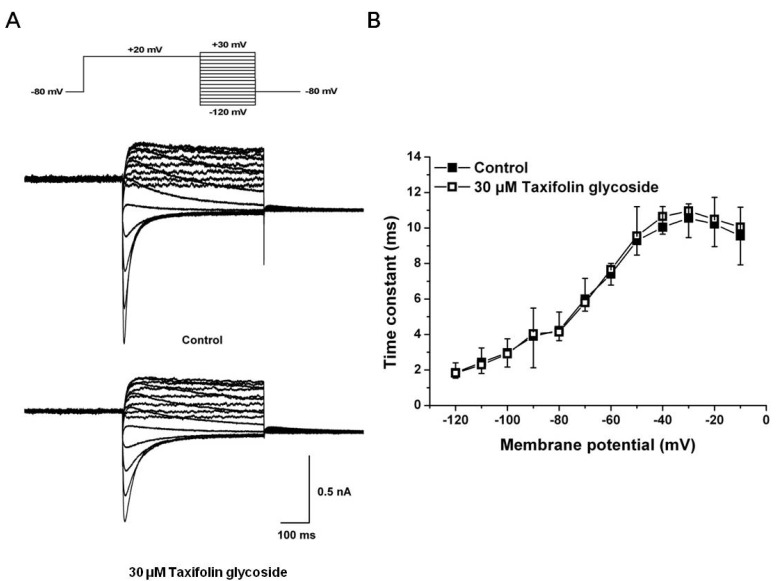
- Taxifolin glycoside is a new drug candidate for the treatment of atopic dermatitis (AD). Many drugs cause side effects such as long QT syndrome by blocking the human ether-a-go-go related gene (hERG) K+ channels. To determine whether taxifolin glycoside would block hERG K+ channels, we recorded hERG K+ currents using a whole-cell patch clamp technique. We found that taxifolin glycoside directly blocked hERG K+ current in a concentration-dependent manner (EC50=9.6+/-0.7 microM). The activation curve of hERG K+ channels was negatively shifted by taxifolin glycoside. In addition, taxifolin glycoside accelerated the activation time constant and reduced the onset of the inactivation time constant. These results suggest that taxifolin glycoside blocks hERG K+ channels that function by facilitating activation and inactivation process.
Figure
Reference
-
2. Takahashi H, Hirata S, Minami H, Fukuyama Y. Triterpene and flavanone glycoside from Rhododendron simsii. Phytochemistry. 2001; 56:875–879. PMID: 11324921.
Article3. Kim CM, Shin MK, Ahn DK, Lee KS. An unabridged dictionary of Chinese herbs. 1998. Jeongdam Publication;p. 1464–1472.4. Kim YJ, Choi SE, Lee MW, Lee CS. Taxifolin glycoside inhibits dendritic cell responses stimulated by lipopolysaccharide and lipoteichoic acid. J Pharm Pharmacol. 2008; 60:1465–1472. PMID: 18957167.
Article5. Ahn JY, Choi SE, Jeong MS, Park KH, Moon NJ, Joo SS, Lee CS, Choi YW, Li K, Lee MK, Lee MW, Seo SJ. Effect of taxifolin glycoside on atopic dermatitis-like skin lesions in NC/Nga mice. Phytother Res. 2010; 24:1071–1077. PMID: 20041431.
Article6. Kang MJ, Eum JY, Park SH, Kang MH, Park KH, Choi SE, Lee MW, Kang KH, Oh CH, Choi YW. Pep-1 peptide-conjugated elastic liposomal formulation of taxifolin glycoside for the treatment of atopic dermatitis in NC/Nga mice. Int J Pharm. 2010; 402:198–204. PMID: 20888893.
Article7. Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999; 354:1625–1633. PMID: 10560690.
Article8. Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010; 62:760–781. PMID: 21079043.
Article9. Cavero I, Mestre M, Guillon JM, Crumb W. Drugs that prolong QT interval as an unwanted effect: assessing their likelihood of inducing hazardous cardiac dysrhythmias. Expert Opin Pharmacother. 2000; 1:947–973. PMID: 11249502.
Article10. Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004; 94:1418–1428. PMID: 15192037.11. Lee SH, Hahn SJ, Min G, Kim J, Jo SH, Choe H, Choi BH. Inhibitory actions of HERG currents by the immunosuppressant drug cyclosporin a. Korean J Physiol Pharmacol. 2011; 15:291–297. PMID: 22128262.
Article12. Lee HA, Kim KS, Hyun SA, Park SG, Kim SJ. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. Korean J Physiol Pharmacol. 2012; 16:327–332. PMID: 23118556.
Article13. Zhao XL, Qi ZP, Fang C, Chen MH, Lv YJ, Li BX, Yang BF. HERG K+ channel blockade by the novel antiviral drug sophocarpine. Biol Pharm Bull. 2008; 31:627–632. PMID: 18379053.14. Towart R, Linders JT, Hermans AN, Rohrbacher J, van der, Ercken M, Cik M, Roevens P, Teisman A, Gallacher DJ. Blockade of the IKs potassium channel: an overlooked cardiovascular liability in drug safety screening? J Pharmacol Toxicol Methods. 2009; 60:1–10. PMID: 19439185.15. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003; 58:32–45. PMID: 12667944.
Article16. Neubig RR, Spedding M, Kenakin T, Christopoulos A. International union of pharmacology committee on receptor nomenclature and drug classification. International union of pharmacology committee on receptor nomenclature and drug classification XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003; 55:597–606. PMID: 14657418.17. Recanatini M, Poluzzi E, Masetti M, Cavalli A, De Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005; 25:133–166. PMID: 15389727.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of the Human Ether-a-go-go-related Gene (HERG) K+ Channels by Lindera erythrocarpa
- Quercetin-3-Methyl Ether Induces Early Apoptosis to Overcome HRV1B Immune Evasion, Suppress Viral Replication, and Mitigate Inflammatory Pathogenesis
- Acepromazine inhibits hERG potassium ion channels expressed in human embryonic kidney 293 cells
- Clinical Experiences with Hiccups during Anesthesia
- Effects of Paroxetine on a Human Ether-a-go-go-related Gene (hERG) K⺠Channel Expressed in Xenopus Oocytes and on Cardiac Action Potential