J Korean Assoc Oral Maxillofac Surg.
2013 Jun;39(3):120-126. 10.5125/jkaoms.2013.39.3.120.
Porcine study on the efficacy of autogenous tooth bone in the maxillary sinus
- Affiliations
-
- 1Division of Oral and Maxillofacial Surgery, Department of Dentistry, Ajou University School of Medicine, Suwon, Korea. arcady@ajou.ac.kr
- KMID: 1430479
- DOI: http://doi.org/10.5125/jkaoms.2013.39.3.120
Abstract
OBJECTIVES
This study sought to elucidate the effect of autogenous tooth bone material by experimenting on minipig's maxillary sinus and performing histological and histomorphometric analyses.
MATERIALS AND METHODS
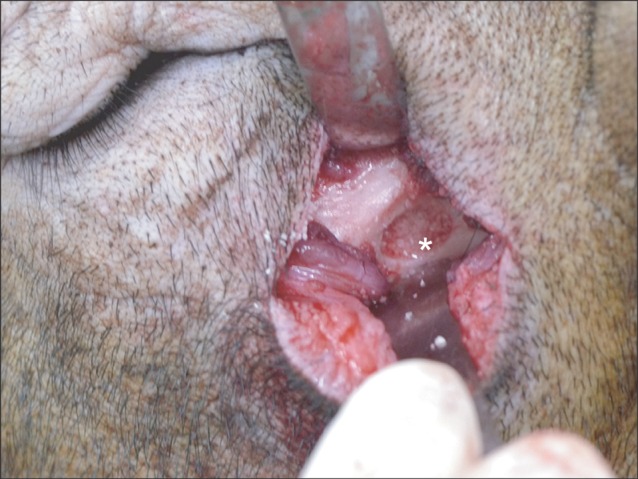
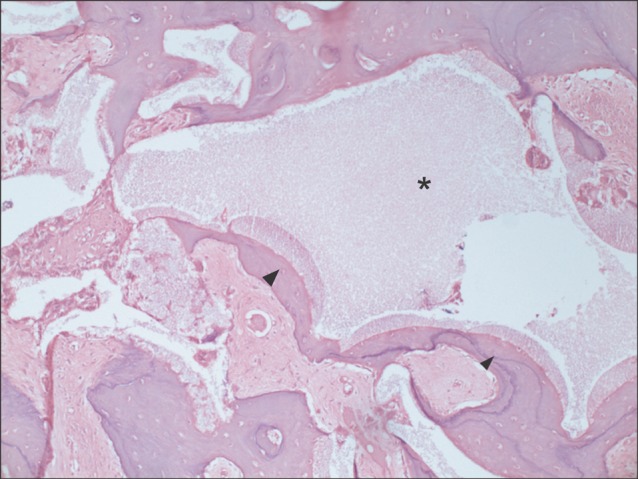
Five 18-24 month-old male minipigs were selected, and right maxillary sinuses were grafted with bone graft material made of their respective autogenous teeth extracted eight weeks earlier. The left sides were grafted with synthetic hydroxyapatite as control groups. All minipigs were sacrificed at 12 weeks after bone graft, which was known to be 1 sigma (sigma) period for pigs. Specimens were evaluated histologically under a light microscope after haematoxylin-eosin staining followed by semi-quantitative study via histomorphometric analysis. The ratio of new bone to total area was evaluated using digital software for calculation of area.
RESULTS
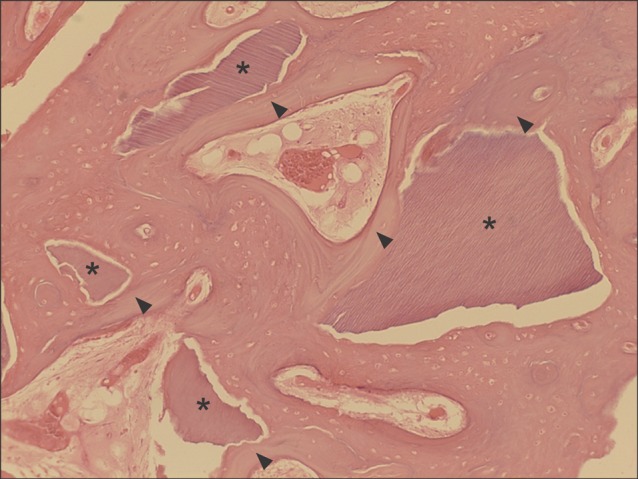
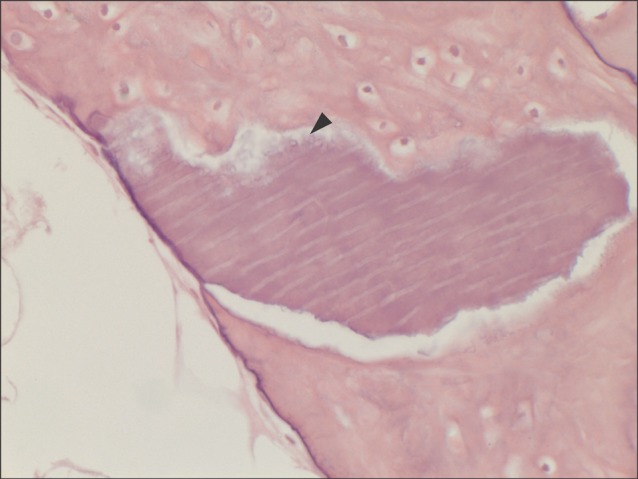
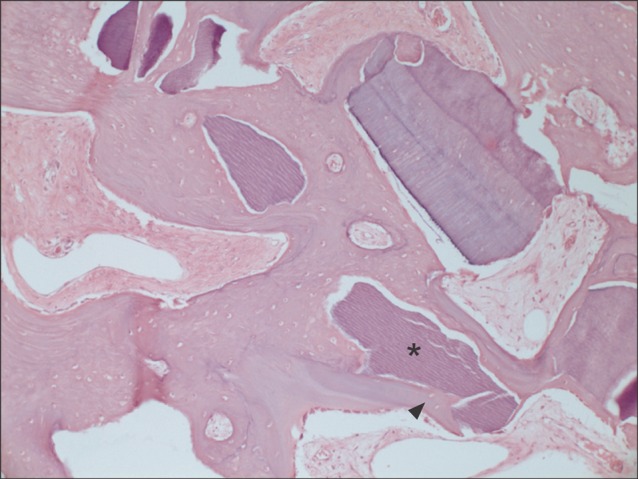
All specimens were available, except one on the right side (experimental group), which was missing during specimen preparation. This study demonstrated new bone at the periphery of the existing bone in both groups, showing evidence of bone remodeling, however, encroachment of new bone on the central part of the graft at the 1 sigma period was observed only in the autogenous tooth bone group (experimental group). Histomorphometric analysis showed more new bone formation in the experimental group compared to the control group. Although the difference was not statistically significant (P>0.05), the mean percentage area for new bone for the experimental and control groups were 57.19%+/-11.16% and 34.07%+/-13.09%, respectively.
CONCLUSION
The novel bone graft material using autogenous tooth is a good alternative to autogenous bone, comparable to autogenous bone, and outperforming synthetic hydroxyapatite bone graft materials in terms of bone regeneration capacity. Augmentation with autogenous tooth bone materials will reduce donor site morbidity without hampering the safety of the autogenous bone graft.
MeSH Terms
Figure
Cited by 1 articles
-
Autogenous tooth bone graft block for sinus augmentation with simultaneous implant installation: a technical note
Kwang-Ho Lee, Young-Kyun Kim, Woo-Jin Cho, In-Woong Um, Masaru Murata, Masaharu Mitsugi
J Korean Assoc Oral Maxillofac Surg. 2015;41(5):284-289. doi: 10.5125/jkaoms.2015.41.5.284.
Reference
-
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–616. PMID: 6993637.2. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants. 1998; 13(Suppl):11–45. PMID: 9715571.3. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986; 30:207–229. PMID: 3516738.4. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503. PMID: 20060336.
Article5. Min BM. Oral biochemistry. 1st ed. Seoul: Daehan Narae Publishing;2007.6. Orban BJ, Bhaskar SN. Orban's oral histology and embryology. 9th ed. St. Louis: Mosby;1980.7. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967; 12:999–1008. PMID: 4226721.
Article8. Kim SG, Yeo HH, Kim YK. Grafting of large defects of the jaws with a particulate dentin-plaster of paris combination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:22–25. PMID: 10442940.9. Park SS, Kim SG, Lim SC, Ong JL. Osteogenic activity of the mixture of chitosan and particulate dentin. J Biomed Mater Res A. 2008; 87:618–623. PMID: 18186071.
Article10. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170. PMID: 7246093.11. Molly L. Bone density and primary stability in implant therapy. Clin Oral Implants Res. 2006; 17(Suppl 2):124–135. PMID: 16968388.
Article12. Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis-Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent. 1998; 18:528–543. PMID: 10321168.13. Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, et al. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999; 14:853–858. PMID: 10612923.14. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983; (174):28–42. PMID: 6339139.
Article15. Hatano N, Shimizu Y, Ooya K. Aclinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004; 15:339–345. PMID: 15142097.16. Kim YK, Kim SG, Lee BG. Bone graft and implant. Vol. 2-2. Seoul: Narae Pub;2007. p. 243–258.17. Lee JK. Outfracture osteotomy on lateral maxillary wall as a modified sinus graft technique. J Oral Maxillofac Surg. 2010; 68:1639–1641. PMID: 20417015.
Article18. Song SI, Jeong HR, Kim HM, Lee JK. Clinical investigation on the feasibility of outfracture osteotomy sinus graft technique. J Korean Assoc Oral Maxillofac Surg. 2009; 35:367–371.20. Kawai T, Urist MR. Bovine tooth-derived bone morphogenetic protein. J Dent Res. 1989; 68:1069–1074. PMID: 2808865.
Article21. Butler WT, Mikulski A, Urist MR, Bridges G, Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977; 56:228–232. PMID: 265954.
Article22. Conover MA, Urist MR. Transmembrane bone morphogenesis by implants of dentin matrix. J Dent Res. 1979; 58:1911. PMID: 290656.
Article23. McKee MD, Aoba T, Moreno EC. Morphology of the enamel organ in the miniature swine. Anat Rec. 1991; 230:97–113. PMID: 2064032.
Article24. Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000; 407:86–90. PMID: 10993078.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maxillary Sinus Augmentation Using Autogenous Teeth: Preliminary Report
- Sinus floor augmentation at the time of tooth removal
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone
- Clinical Study on the Efficacy of the Autogenous Tooth Bone Graft Material (AutoBT)
- A Double Layers Technique for Maxillary Sinus Augmentation with Demineralized and Mineralized Bone Graft Materials