J Korean Med Sci.
2013 Feb;28(2):274-279. 10.3346/jkms.2013.28.2.274.
Comparison of Immune Response by Virus Infection and Vaccination to 2009 Pandemic Influenza A/H1N1 in Children
- Affiliations
-
- 1Department of Pediatrics, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea. kdh281920@gmail.com
- KMID: 1429198
- DOI: http://doi.org/10.3346/jkms.2013.28.2.274
Abstract
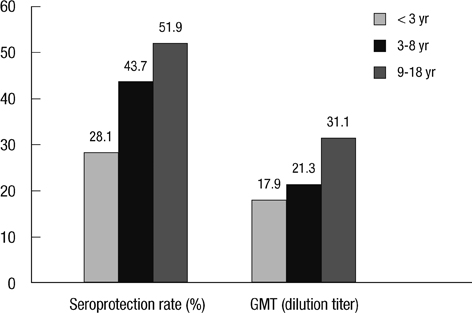
- We aimed to compare the immune response induced by natural infection with 2009 pandemic influenza A/H1N1 (pH1N1) virus and by monovalent pH1N1 vaccination in children and adolescents. This cross-sectional clinical study was conducted at 3 hospitals in Korea from February to May 2010. A total of 266 healthy subjects aged from 6 months to 18 yr were tested for the presence of the antibody against pH1N1 using hemagglutination inhibition (HI) test. Information about pH1N1 vaccination and laboratory-confirmed pH1N1 infection history was obtained. The overall rate of HI titers of > or = 1:40 against pH1N1 was 38.7%, and the geometric mean titer (GMT) was 20.5. Immunogenicity of pH1N1 vaccination only was reflected by a 41.1% of seroprotection rate and a GMT of 22.5. Immunogenicity of natural infection only was reflected by a 61.0% of seroprotection rate and a GMT of 40.0. GMT was significantly higher in the subjects of natural infection group than in the subjects of pH1N1 vaccination group (P < 0.001). The immune responses induced by natural pH1N1 infection exceed those induced by pH1N1 vaccinations.
MeSH Terms
-
Adolescent
Antibodies, Neutralizing/blood
Antibody Formation
Child
Child, Preschool
Cross-Sectional Studies
Hemagglutination Inhibition Tests
Humans
Infant
Influenza A Virus, H1N1 Subtype/*immunology/metabolism
Influenza, Human/epidemiology/*immunology/prevention & control
Pandemics
Vaccination
Antibodies, Neutralizing
Figure
Reference
-
1. CDC. Swine influenza A (H1N1) infection in two children-Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:400–402.2. CDC. Outbreak of swine origin influenza A (H1N1) virus infection-Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:467–470.3. Kreijtz JH, Fouchier RA, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011. 162:19–30.4. Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000. 18:957–1030.5. Broberg E, Nicoll A, Amato-Gauci A. Seroprevalence to influenza A (H1N1) 2009 virus: where are we? Clin Vaccine Immunol. 2011. 18:1205–1212.6. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010. 375:1100–1108.7. Kim JH, Yoo HS, Lee JS, Lee EG, Park HK, Sung YH, Kim S, Kim HS, Shin SY, Lee JK. The spread of pandemic H1N1 2009 by age and region and the comparison among monitoring tools. J Korean Med Sci. 2010. 25:1109–1112.8. Von Kries R, Weiss S, Falkenhorst G, Wirth S, Kaiser P, Huppertz HI, Tenenbaum T, Schroten H, Streng A, Liese J, et al. Post-pandemic seroprevalence of pandemic influenza A (H1N1) 2009 infection (Swine flu) among children <18 years in Germany. PLoS One. 2011. 6:e23955.9. Korea Centers for Disease Control and Prevention. Current status of selected infectious diseases. Public Health Wkly Rep. 2009. 2:886.10. WHO. WHO manual on animal influenza diagnosis and surveillance. accessed on 27 Nov 2011. Available at: www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf.11. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979. 35:69–75.12. Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, Nissen M, Marshall H, Booy R, Heron L, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010. 303:37–46.13. Plennevaux E, Sheldon E, Blatter M, Reeves-Hoché MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010. 375:41–48.14. Oh CE, Lee J, Kang JH, Hong YJ, Kim YK, Cheong HJ, Ahn YJ, Kim SH, Lee HJ. Safety and immunogenicity of an inactivated split-virus influenza A/H1N1 vaccine in healthy children from 6 months to <18 years of age: a prospective, open-label, multi-center trial. Vaccine. 2010. 28:5857–5863.15. Fiore AE, Neuzil KM. 2009 influenza A (H1N1) monovalent vaccines for children. JAMA. 2010. 303:73–74.16. Walter EB, Rajagopal S, Zhu Y, Neuzil KM, Fairchok MP, Englund JA. Trivalent inactivated influenza vaccine (TIV) immunogenicity in children 6 through 23 months of age: do children of all ages respond equally? Vaccine. 2010. 28:4376–4383.17. Wright P. Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Influenza viruses. Nelson textbook of pediatrics. 2007. 18th ed. Philadelphia, PA: Saunders;1384–1386.18. Takahashi E, Kataoka K, Fujii K, Chida J, Mizuno D, Fukui M, Hiro-O Ito, Fujihashi K, Kido H. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect. 2010. 12:778–783.19. Li IW, Hung IF, To KK, Chan KH, Wong SS, Chan JF, Cheng VC, Tsang OT, Lai ST, Lau YL, et al. The natural viral load profile of patients with pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment. Chest. 2010. 137:759–768.20. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009. 361:1945–1952.21. Jackson ML, France AM, Hancock K, Lu X, Veguilla V, Sun H, Liu F, Hadler J, Harcourt BH, Esposito DH, et al. Serologically confirmed household transmission of 2009 pandemic influenza A (H1N1) virus during the first pandemic wave-New York City, April-May 2009. Clin Infect Dis. 2011. 53:455–462.22. Chao DY, Cheng KF, Hsieh YH, Li TC, Wu TN, Chen CY, Tsai CA, Chen JH, Chiu HT, Lu JJ, et al. Serological response and persistence in schoolchildren with high baseline seropositive rate after receiving 2009 pandemic influenza A (H1N1) vaccine. Vaccine. 2011. 29:617–623.23. Choi YS, Baek YH, Kang W, Nam SJ, Lee J, You S, Chang DY, Youn JC, Choi YK, Shin EC. Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin Vaccine Immunol. 2011. 18:1519–1523.24. Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, Rosella LC, Dickinson JA, Gilca R, Sethi P, et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010. 7:e1000258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The 2009 H1N1 Pandemic Influenza in Korea
- Influenza Associated Pneumonia
- Status of and Factors Influencing Vaccination against the Pandemic (H1N1) 2009 Virus among University Students from the Fields of Nursing and Allied Health
- Epidemiology, clinical manifestations, and management of pandemic novel Influenza A (H1N1)
- Novel Influenza A/H1N1 Pandemic: Current Status and Prospects