Ann Lab Med.
2012 May;32(3):190-193. 10.3343/alm.2012.32.3.190.
Comparison of Serum Zinc Levels Measured by Inductively Coupled Plasma Mass Spectrometry in Preschool Children with Febrile and Afebrile Seizures
- Affiliations
-
- 1Department of Pediatrics, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Laboratory Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. docto95@nate.com
- KMID: 1381683
- DOI: http://doi.org/10.3343/alm.2012.32.3.190
Abstract
- BACKGROUND
Changes in levels of trace elements have been proposed to underlie febrile seizures. Particularly, low zinc levels have been proposed as related factor of febrile seizure. In this study, we investigated whether mean serum zinc levels differed between children with febrile seizure and afebrile seizure.
METHODS
Using inductively coupled plasma mass spectrometry, serum zinc levels were measured in 288 children who had been diagnosed with febrile seizures (N=248) and afebrile seizures (N=40). Mean serum zinc levels were compared between the 2 groups.
RESULTS
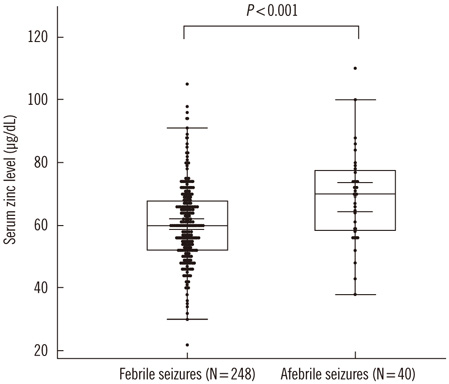
Mean serum zinc level was 60.5+/-12.7 microg/dL in the febrile seizure group and 68.9 +/-14.5 microg/dL in the afebrile seizure group. A significant difference in serum zinc levels was observed between the febrile and afebrile seizure groups (P<0.001).
CONCLUSIONS
Zinc levels in children with febrile seizure were significantly lower than those in children with afebrile seizure.
Keyword
MeSH Terms
Figure
Reference
-
1. Martindale JL, Goldstein JN, Pallin DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am. 2011. 29:15–27.
Article2. Siqueira LF. Febrile seizures: update on diagnosis and management. Rev Assoc Med Bras. 2010. 56:489–492.3. Liscák R, Vladyka V, Simonová G, Vymazal J, Novotny J Jr. Gamma knife surgery of brain cavernous hemangiomas. J Neurosurg. 2005. 102:Suppl. 207–213.
Article4. Guerreiro MM. Treatment of febrile seizures. J Pediatr (Rio J). 2002. 78(Suppl 1):S9–S13.
Article5. Consensus statement. Febrile seizures: long-term management of children with fever-associated seizures. Pediatrics. 1980. 66:1009–1012.6. Mollah MA, Dey PR, Tarafdar SA, Akhter S, Ahmed S, Hassan T, et al. Zinc in CSF of patients with febrile convulsion. Indian J Pediatr. 2002. 69:859–861.
Article7. Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006. 35:165–172.
Article8. Nakayama J, Arinami T. Molecular genetics of febrile seizures. Epilepsy Res. 2006. 70(Suppl 1):S190–S198.
Article9. Haspolat S, Mihçi E, Coşkun M, Gümüslü S, Ozben T, Ye in O. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J Child Neurol. 2002. 17:749–751.10. Castilla-Guerra L, del Carmen Fernández-Moreno M, López-Chozas JM, Fernández-Bolaños R. Electrolytes disturbances and seizures. Epilepsia. 2006. 47:1990–1998.
Article11. Vallee BL, Falchuk KH. Zinc and gene expression. Philos Trans R Soc Lond B Biol Sci. 1981. 294:185–197.12. Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989. 31:145–238.
Article13. Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996. 271:369–373.
Article14. Gibbs JW 3rd, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997. 77:1924–1938.
Article15. Shumate MD, Lin DD, Gibbs JW 3rd, Holloway KL, Coulter DA. GABA(A) receptor function in epileptic human dentate granule cells: comparison to epileptic and control rat. Epilepsy Res. 1998. 32:114–128.
Article16. Ganesh R, Janakiraman L. Serum zinc levels in children with simple febrile seizure. Clin Pediatr (Phila). 2008. 47:164–166.
Article17. Garty BZ, Olomucki R, Lerman-Sagie T, Nitzan M. Cerebrospinal fluid zinc concentrations in febrile convulsions. Arch Dis Child. 1995. 73:338–341.
Article18. Yamashiro O, Morimoto A, Sakata Y, Watanabe T, Murakami N. Febrile and metabolic tolerance to endotoxin and human recombinant interleukin-1 beta in rabbits. Am J Physiol. 1993. 264:R1180–R1185.
Article19. van Miert AS, van Duin CT, Wensing T. Fever and acute phase response induced in dwarf goats by endotoxin and bovine and human recombinant tumour necrosis factor alpha. J Vet Pharmacol Ther. 1992. 15:332–342.
Article20. Sakata Y, Morimoto A, Long NC, Murakami N. Fever and acute-phase response induced in rabbits by intravenous and intracerebroventricular injection of interleukin-6. Cytokine. 1991. 3:199–203.
Article21. Van Miert AS, Van Duin CT, Wensing T. Fever and changes in plasma zinc and iron concentrations in the goat. The effects of interferon inducers and recombinant IFN-alpha 2a. J Comp Pathol. 1990. 103:289–300.22. Morimoto A, Murakami N, Nakamori T, Sakata Y, Watanabe T. Brain regions involved in the development of acute phase responses accompanying fever in rabbits. J Physiol. 1989. 416:645–657.
Article23. Van Miert AS, Van Duin CT, Verheijden JH, Schotman AJ, Nieuwenhuis J. Fever and changes in plasma zinc and iron concentrations in the goat: the role of leukocytic pyrogen. J Comp Pathol. 1984. 94:543–557.
Article24. Gündüz Z, Yavuz I, Koparal M, Kumanda S, Saraymen R. Serum and cerebrospinal fluid zinc levels in children with febrile convulsions. Acta Paediatr Jpn. 1996. 38:237–241.25. Amiri M, Farzin L, Moassesi ME, Sajadi F. Serum trace element levels in febrile convulsion. Biol Trace Elem Res. 2010. 135:38–44.
Article26. Tütüncüoğlu S, Kütükçüler N, Kepe L, Coker C, Berdeli A, Tekgül H. Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int. 2001. 43:235–239.
Article27. Lin CN, Wilson A, Church BB, Ehman S, Roberts WL, McMillin GA. Pediatric reference intervals for serum copper and zinc. Clin Chim Acta. 2012. 413:612–615.
Article28. Macours P, Aubry JC, Hauquier B, Boeynaems JM, Goldman S, Moreno-Reyes R. Determination of urinary iodine by inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. 2008. 22:162–165.
Article29. Caldwell KL, Maxwell CB, Makhmudov A, Pino S, Braverman LE, Jones RL, et al. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem. 2003. 49:1019–1021.
Article30. May SL, May WA, Bourdoux PP, Pino S, Sullivan KM, Maberly GF. Validation of a simple, manual urinary iodine method for estimating the prevalence of iodine-deficiency disorders, and interlaboratory comparison with other methods. Am J Clin Nutr. 1997. 65:1441–1445.
Article31. Miksa IR, Buckley CL, Carpenter NP, Poppenga RH. Comparison of selenium determination in liver samples by atomic absorption spectroscopy and inductively coupled plasma-mass spectrometry. J Vet Diagn Invest. 2005. 17:331–340.
Article32. Barnes RM. Analytical plasma source mass spectrometry in biomedical research. Anal Bioanal Chem. 1996. 355:433–441.
Article33. Lee SY, Oh HJ, Choi YH, Kim JW, Kim SH. Trace metal analysis using inductively coupled plasma-mass spectrometry (ICP-MS). Korean J Lab Med. 2004. 24:362–370.34. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006. 20:3–18.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Recurrence in Febrile Seizures and Serum Zinc Levels
- Pathogenesis and Correlations of Serum and Cerebrospinal Fluid Zinc Levels in Febrile Convulsions
- Serum Zinc Levels in Young Children with Recurrent Wheeze
- Hair Zinc and Lead : Relationship to Nutrient Intake and Height and Body Weight in Korean Preschool Children
- The Influence of Low Serum Sodium Levels on the Risk of Repeated Febrile Convulsions during the Same Febrile Illness