Ann Lab Med.
2012 Mar;32(2):145-152. 10.3343/alm.2012.32.2.145.
Evaluation of Recombinant Factor VIIa Treatment for Massive Hemorrhage in Patients with Multiple Traumas
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University School of Medicine, Busan, Korea. hhkim@pusan.ac.kr
- 2Department of Emergency Medicine, Pusan National University School of Medicine, Busan, Korea.
- 3BioMedical Research Institute, Pusan National University School of Medicine, Busan, Korea.
- KMID: 1245255
- DOI: http://doi.org/10.3343/alm.2012.32.2.145
Abstract
- BACKGROUND
Recent studies and case reports have shown that recombinant factor VIIa (rFVIIa) treatment is effective for reversing coagulopathy and reducing blood transfusion requirements in trauma patients with life-threatening hemorrhage. The purpose of this study is to evaluate the effect of rFVIIa treatment on clinical outcomes and cost effectiveness in trauma patients.
METHODS
Between January 2007 and December 2010, we reviewed the medical records of patients who were treated with rFVIIa (N=18) or without rFVIIa (N=36) for life-threatening hemorrhage due to multiple traumas at the Emergency Department of Pusan National University Hospital in Busan, Korea. We reviewed patient demographics, baseline characteristics, initial vital signs, laboratory test results, and number of units transfused, and then analyzed clinical outcomes and 24-hr and 30-day mortality rates. Thromboembolic events were monitored in all patients. Transfusion costs and hospital stay costs were also calculated.
RESULTS
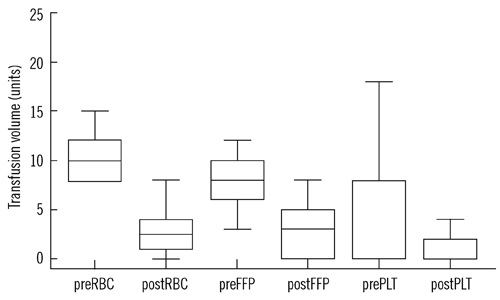
In the rFVIIa-treated group, laboratory test results and clinical outcomes improved, and the 24-hr mortality rate decreased compared to that in the untreated group; however, 30-day mortality rate did not differ between the groups. Thromboembolic events did not occur in both groups. Transfusion and hospital stay costs in the rFVIIa-treated group were cost effective; however, total treatment costs, including the cost of rFVIIa, were not cost effective.
CONCLUSIONS
In our study, rFVIIa treatment was shown to be helpful as a supplementary drug to improve clinical outcomes and reduce the 24-hr mortality rate, transfusion and hospital stay costs, and transfusion requirements in trauma patients with life-threatening hemorrhage.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Factor VIIa/*therapeutic use
Hemoglobins/analysis
Hemorrhage/complications/*drug therapy/mortality
Humans
Middle Aged
Multiple Trauma/*complications
Partial Thromboplastin Time
Platelet Count
Prothrombin Time
Recombinant Proteins/therapeutic use
Retrospective Studies
Treatment Outcome
Figure
Reference
-
1. Miller RD. Complications of massive blood transfusions. Anesthesiology. 1973. 39:82–93.
Article2. Rudolph R, Boyd CR. Massive transfusion: complications and their management. South Med J. 1990. 83:1065–1070.3. Pike AC, Brzozowski AM, Roberts SM, Olsen OH, Persson E. Structure of human factor VIIa and its implications for the triggering of blood coagulation. Proc Natl Acad Sci. 1999. 96:8925–8930.
Article4. Ingerslev J. Efficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitors. Semin Thromb Hemost. 2000. 26:425–432.
Article5. Franchini M. Recombinant factor VIIa: a review on its clinical use. Int J Hematol. 2006. 83:126–138.
Article6. Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999. 354:1879.
Article7. Goodnough LT, Shander AS. Recombinant factor VIIa: safety and efficacy. Curr Opin Hematol. 2007. 14:504–509.
Article8. Joo YM, Yeom SR, Ryu JH, Jeong JW, Kim YI, Min MK, et al. Successful hemostasis by the use of recombinant factor VIIa in uncontrolled active hemorrhage of multiple trauma patients. J Korean Soc Emerg Med. 2011. 22:22–29.9. Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, et al. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001. 51:431–438.
Article10. Dutton RP, Hess JR, Scalea TM. Recombinant factor VIIa for control of hemorrhage: early experience in critically ill trauma patients. J Clin Anesth. 2003. 15:184–188.
Article11. Mayo A, Misgav M, Kluger Y, Greenberg R, Pauzner D, Klausner J, et al. Recombinant activated factor VII (NovoSeven) : addition to replacement therapy in acute, uncontrolled and life-threatening bleeding. Vox Sang. 2004. 87:34–40.12. Goodnough LT, Lublin DM, Zhang L, Despotis G, Eby C. Transfusion medicine service policies for recombinant factor VIIa administration. Transfusion. 2004. 44:1325–1331.
Article13. Loudon B, Smith MP. Recombinant factor VIIa as an adjunctive therapy for patients requiring large volume transfusion: a pharmacoeconomic evaluation. Intern Med J. 2005. 35:463–467.
Article14. Grounds RM, Seebach C, Knothe C, Paluszkiewicz P, Smith TS, Kasal E, et al. Use of recombinant activated factor VII (Novoseven) in trauma and surgery: analysis of outcomes reported to an international registry. J Intensive Care Med. 2006. 21:27–39.
Article15. Rizoli SB, Nascimento B Jr, Osman F, Netto FS, Kiss A, Callum J, et al. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006. 61:1419–1425.
Article16. Cameron P, Philips L, Balogh Z, Joseph A, Pearce A, Parr M, et al. The use of recombinant activated factor VII in trauma patients: Experience from the Australian and New Zealand haemostasis registry. Injury. 2007. 38:1030–1038.
Article17. Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005. 59:8–15.
Article18. Rizoli SB, Boffard KD, Riou B, Warren B, Lau P, Kluger Y, et al. Recombinant activated factor VII as an adjunctive therapy for bleeding control in severe trauma patients with coagulopathy: subgroup analysis from two randomized trials. Crit Care. 2006. 10:R178.19. Martinowitz U, Michaelson M. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli multidisciplinary rFVIIa task force. J Thromb Haemost. 2005. 3:640–648.
Article20. Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding - a European perspective. Crit Care. 2006. 10:R120.21. Klitgaard T, Tabanera y Palacios R, Boffard KD, Iau PT, Warren B, Rizoli S, et al. Pharmacokinetics of recombinant activated factor VII in trauma patients with severe bleeding. Crit Care. 2006. 10:R104.22. Yank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011. 154:529–540.
Article23. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2011. CD005011.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant Factor VIIa Treatment for Acute Intracerebral Hemorrhage
- The prophylactic use of recombinant factor VIIa in a patient with DeBakey type III aortic dissection: A case report
- Diffuse alveolar hemorrhage and recombinant factor VIIa treatment in pediatric patients
- A Case of Successful Treatment of Childhood Intractable Gastrointestinal Hemorrhage with Low Dose Recombinant Activated Factor VII (NovoSeven (R))
- Clinical Efficacy of Recombinant Activated Factor VII in Management of Postpartum Hemorrhage