J Korean Med Sci.
2007 Aug;22(4):621-628. 10.3346/jkms.2007.22.4.621.
Diagnostic Value of Galectin-3, HBME-1, Cytokeratin 19, High Molecular Weight Cytokeratin, Cyclin D1 and p27(kip1) in the Differential Diagnosis of Thyroid Nodules
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. sypmd@snubh.org
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1127076
- DOI: http://doi.org/10.3346/jkms.2007.22.4.621
Abstract
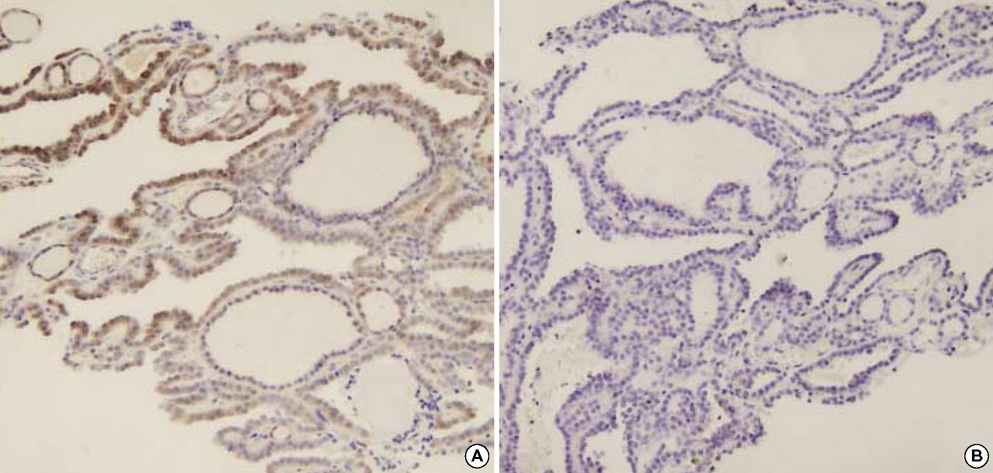
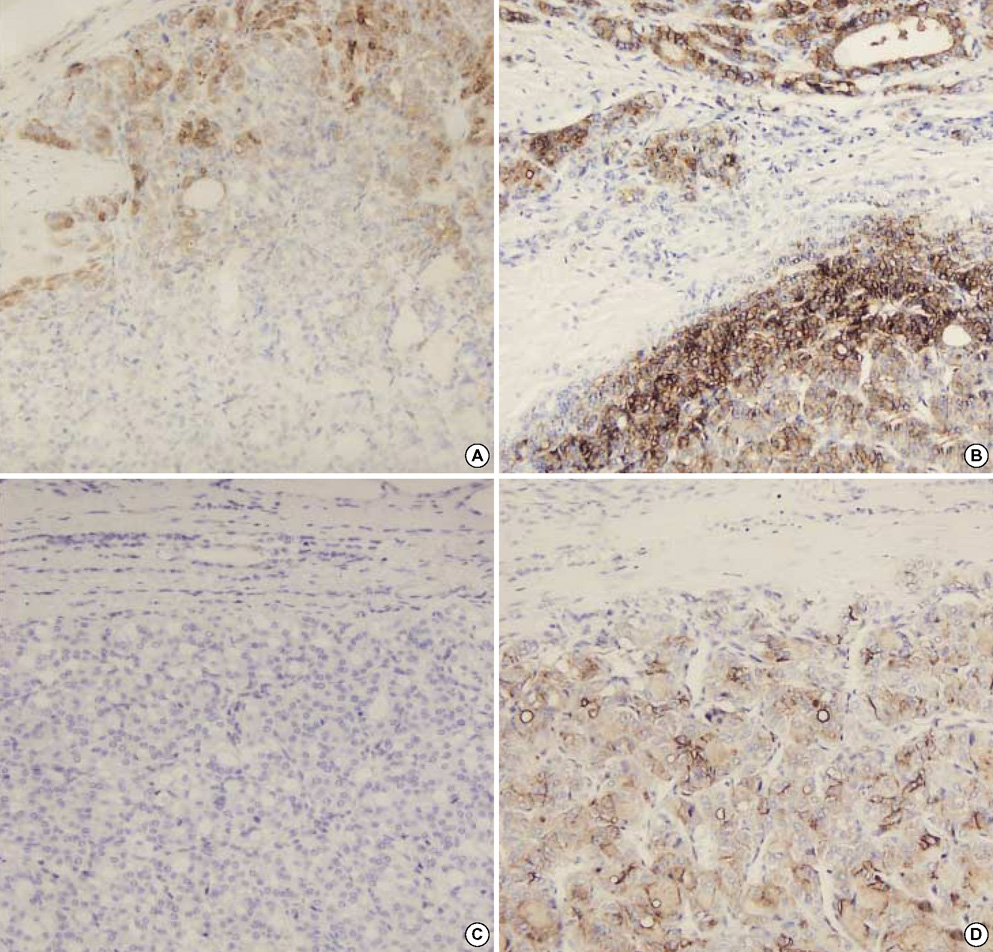
- The distinction between benign and malignant thyroid tumors is critical for the management of patients with thyroid nodules. We applied immunohistochemical staining for galectin-3, HBME-1, cytokeratin 19 (CK19), high molecular weight cytokeratin (HMWCK), cyclin D1 and p27(kip1) in 295 thyroid lesions to determine their diagnostic accuracy. The expression of all markers was significantly associated with differentiated thyroid carcinoma (DTC).The sensitivity for the diagnosis of DTC was 94.7% with galectin-3, 91.3% with HBME-1, and 90.3% with CK19. The specificities of these markers were 95.5%, 69.7%, and 83.1%, respectively. Combining these markers, co-expression of galectin-3 and CK19 or galectin-3 and HBME-1 was seen in 93.2% of carcinomas but in none of the benign nodules. Comparing follicular variant of papillary carcinoma (FVPC) with follicular carcinoma (FC), the expression of galectin-3, CK19, and HMWCK was significantly higher in FVPC. When comparing FC with FA, the expression of galectin-3 and HBME-1 was significantly higher in FC. These results suggest that 1) galectin-3 is a useful marker in the distinction between benign and malignant thyroid tumors, 2) the combined use of HBME-1 and CK19 can increase the diagnostic accuracy, and 3) the use of CK19 and HMWCK can aid in the differential diagnosis between PC and FC.
Keyword
MeSH Terms
-
Adenocarcinoma, Follicular/diagnosis/metabolism
Carcinoma, Papillary, Follicular/diagnosis/metabolism
Cyclin D1/analysis
Cyclin-Dependent Kinase Inhibitor p27/analysis
Diagnosis, Differential
Galectin 3/analysis
Humans
Immunohistochemistry
Intracellular Signaling Peptides and Proteins/analysis
Keratin-19/analysis
Keratins/analysis
Sensitivity and Specificity
Thyroid Gland/chemistry/*pathology
Thyroid Nodule/*diagnosis/metabolism
Tumor Markers, Biological/*analysis
Figure
Cited by 1 articles
-
The Diagnostic Usefulness of HMGA2, Survivin, CEACAM6, and SFN/14-3-3 δ in Follicular Thyroid Carcinoma
Min Hye Jang, Kyeong Cheon Jung, Hye Sook Min
J Pathol Transl Med. 2015;49(2):112-117. doi: 10.4132/jptm.2015.01.31.
Reference
-
1. Rosai J, Carcangiu ML, DeLellis RA. Rosai J, Sobin LH, editors. Tumors of the thyroid gland. Atlas of tumor pathology. 1992. Washington, DC: Armed Forces Institute of Pathology;3rd series. Fasc. 5.2. Saxen E, Franssila K, Bjarnason O, Normann T, Ringertz N. Observer variation in histologic classification of thyroid cancer. Acta Pathol Microbiol Scand [A]. 1978. 86A:483–486.3. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, Tsujimoto M, Kakudo K. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002. 26:1508–1514.
Article4. Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004. 9:305–328.5. Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, Cappia S, De Giuli P, Angeli A. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998. 58:3015–3020.6. Gasbarri A, Martegani MP, Del Prete F, Lucante T, Natali PG, Bartolazzi A. Galectin-3 and CD44v6 isoforms in the preoperative evaluation of thyroid nodules. J Clin Oncol. 1999. 17:3494–3502.
Article7. Kawachi K, Matsushita Y, Yonezawa S, Nakano S, Shirao K, Natsugoe S, Sueyoshi K, Aikou T, Sato E. Galectin-3 expression in various thyroid neoplasms and its possible role in metastasis formation. Hum Pathol. 2000. 31:428–433.
Article8. Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orlandi F, Nardi F, Vecchione A, Tecce R, Larsson O. Thyroid Cancer Study Group. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet. 2001. 357:1644–1650.
Article9. Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM, Baloch ZW. Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med. 2002. 126:710–713.
Article10. Volante M, Bozzalla-Cassione F, Orlandi F, Papotti M. Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch. 2004. 444:309–312.
Article11. Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005. 18:48–57.
Article12. de Matos PS, Ferreira AP, de Oliveira Facuri F, Assumpcao LV, Metze K, Ward LS. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005. 47:391–401.
Article13. Park MI, Kang DY. Usefulness of galectin-3, cytokeratin 19, p53, and Ki-67 for the differential diagnosis of thyroid tumors. Korean J Pathol. 2006. 40:86–92.14. Martins L, Matsuo SE, Ebina KN, Kulcsar MA, Friguglietti CU, Kimura ET. Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J Clin Endocrinol Metab. 2002. 87:4806–4810.
Article15. Mehrotra P, Okpokam A, Bouhaidar R, Johnson SJ, Wilson JA, Davies BR, Lennard TW. Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology. 2004. 45:493–500.
Article16. Sheibani K, Esteban JM, Bailey A, Battifora H, Weiss LM. Immunopathologic and molecular studies as an aid to the diagnosis of malignant mesothelioma. Hum Pathol. 1992. 23:107–116.
Article17. Miettinen M, Karkkainen P. Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity with malignant tumours. Virchows Arch. 1996. 429:213–219.18. Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001. 14:338–342.
Article19. Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y, Nakao A. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003. 50:173–177.
Article20. Raphael SJ, McKeown-Eyssen G, Asa SL. High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod Pathol. 1994. 7:295–300.21. Miettinen M, Kovatich AJ, Karkkainen P. Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch. 1997. 431:407–413.22. Sahoo S, Hoda SA, Rosai J, DeLellis RA. Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma: a note of caution. Am J Clin Pathol. 2001. 116:696–702.23. Liberman E, Weidner N. Papillary and follicular neoplasms of the thyroid gland. Differential immunohistochemical staining with high-molecular-weight keratin and involucrin. Appl Immunohistochem Mol Morphol. 2000. 8:42–48.24. Lazzereschi D, Sambuco L, Carnovale Scalzo C, Ranieri A, Mincione G, Nardi F, Colletta G. Cyclin D1 and Cyclin E expression in malignant thyroid cells and in human thyroid carcinomas. Int J Cancer. 1998. 76:806–811.
Article25. Wang S, Wuu J, Savas L, Patwardhan N, Khan A. The role of cell cycle regulatory proteins, cyclin D1, cyclin E, and p27 in thyroid carcinogenesis. Hum Pathol. 1998. 29:1304–1309.
Article26. Goto A, Sakamoto A, Machinami R. An immunohistochemical analysis of cyclin D1, p53, and p21waf1/cip1 proteins in tumors originating from the follicular epithelium of the thyroid gland. Pathol Res Pract. 2001. 197:217–222.27. Erickson LA, Jin L, Wollan PC, Thompson GB, van Heerden J, Lloyd RV. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998. 11:169–174.28. Resnick MB, Schacter P, Finkelstein Y, Kellner Y, Cohen O. Immunohistochemical analysis of p27/kip1 expression in thyroid carcinoma. Mod Pathol. 1998. 11:735–739.29. Erickson LA, Yousef OM, Jin L, Lohse CM, Pankratz VS, Lloyd RV. p27kip1 expression distinguishes papillary hyperplasia in Graves' disease from papillary thyroid carcinoma. Mod Pathol. 2000. 13:1014–1019.
Article30. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003. 88:2318–2326.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Expression of galectin-3, skp2, p27 and cyclin D1 in Benign and Malignant Thyroid Lesions
- The Usefulness of Immunohistochemical Staining for Diagnosis of Thyroid Nodule in Preoperative Ultrasonography-Guided Core Needle Biopsy
- Expression of p27kip1, Cyclin D1 and p53 Protein in Ductal Carcinoma In Situ of the Breast
- Usefulness of Galectin-3, Cytokeratin 19, p53, and Ki-67 for the Differential Diagnosis of Thyroid Tumors
- CD56 and High Molecular Weight Cytokeratin as Diagnostic Markers of Papillary Thyroid Carcinoma