Korean J Radiol.
2011 Oct;12(5):620-625. 10.3348/kjr.2011.12.5.620.
A Spinal Cord Astrocytoma and Its Concurrent Osteoblastic Metastases at the Time of the Initial Diagnosis: a Case Report and Literature Review
- Affiliations
-
- 1Department of Radiology and the Research Institute of Radiological Science, Gangnam Severance Hospital, Yonsei University, Seoul 135-720, Korea. agn70@yuhs.ac
- 2Department of Pathology, Gangnam Severance Hospital, Yonsei University, Seoul 135-720, Korea.
- 3Department of Nuclear Medicine, Gangnam Severance Hospital, Yonsei University, Seoul 135-720, Korea.
- 4Department of Neurosurgery, the Spine and Spinal Cord Institute, Gangnam Severance Hospital, Yonsei University, Seoul 135-720, Korea.
- 5Department of Orthopedic Surgery, Gangnam Severance Hospital, Yonsei University, Seoul 135-720, Korea.
- KMID: 1116448
- DOI: http://doi.org/10.3348/kjr.2011.12.5.620
Abstract
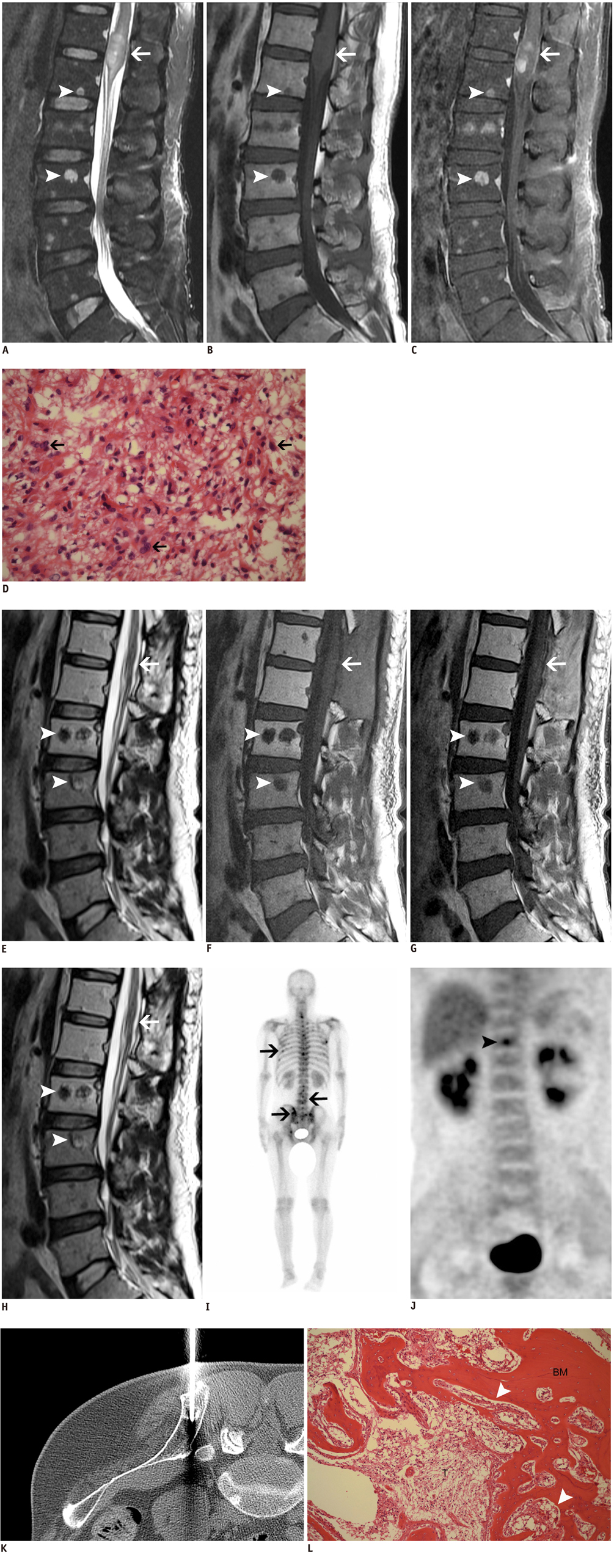
- Bone metastasis from a spinal cord astrocytoma has been reported only twice in the English medical literature. It is generally known that bone metastasis is found after the initial diagnosis with/without intervening surgery rather than being found at the time of the diagnosis of astrocytoma. The purpose of this article is to report for the first time a case of concurrent bone metastasis from a spinal cord astrocytoma at the time of diagnosing the spinal cord astrocytoma.
Keyword
MeSH Terms
Figure
Reference
-
1. Roonprapunt C, Houten JK. Spinal cord astrocytomas: presentation, management, and outcome. Neurosurg Clin N Am. 2006. 17:29–36.2. Newman RP, Schaefer EJ, Thomas CB, Oldfield EH. Abetalipoproteinemia and metastatic spinal cord glioblastoma. Arch Neurol. 1984. 41:554–556.3. Eade OE, Urich H. Metastasising gliomas in young subjects. J Pathol. 1971. 103:245–256.4. Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F, et al. Bone metastasis from glioblastoma multiforme without central nervous system relapse: a case report. Anticancer Res. 2004. 24:2563–2565.5. Beauchesne P, Soler C, Mosnier JF. Diffuse vertebral body metastasis from a glioblastoma multiforme: a technetium-99m Sestamibi single-photon emission computerized tomography study. J Neurosurg. 2000. 93:887–890.6. Rajagopalan V, El Kamar FG, Thayaparan R, Grossbard ML. Bone marrow metastases from glioblastoma multiforme--A case report and review of the literature. J Neurooncol. 2005. 72:157–161.7. Utsuki S, Tanaka S, Oka H, Iwamoto K, Sagiuchi T, Fujii K. Glioblastoma multiforme metastasis to the axis. Case report. J Neurosurg. 2005. 102:540–542.8. Mihara F, Ikeda M, Rothman MI, Numaguchi Y, Kristt D. Vertebral body metastasis of glioblastoma multiforme with epidural mass formation. Contrast-enhanced MRI study. Clin Imaging. 1994. 18:386–389.9. Myers T, Egelhoff J, Myers M. Glioblastoma multiforme presenting as osteoblastic metastatic disease: case report and review of the literature. AJNR Am J Neuroradiol. 1990. 11:802–803.10. Taoka T, Mayr NA, Lee HJ, Yuh WT, Simonson TM, Rezai K, et al. Factors influencing visualization of vertebral metastases on MR imaging versus bone scintigraphy. AJR Am J Roentgenol. 2001. 176:1525–1530.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Intramedullary Spinal Cord Astrocytoma Associated with Neurofibromatosis Type 1
- A Case of Spinal Cord Astrocytoma Associated with Hemorrhage
- Intracranial Dissemination from Spinal Cord Anaplastic Astrocytoma
- Intramedullary Spinal Cord Metastasis: A Report of Two Cases and a Review of the Literature
- Intracranial Metastases of Cervical Intramedullary Low-Grade Astrocytoma without Malignant Transformation in Adult