J Vet Sci.
2010 Jun;11(2):173-175. 10.4142/jvs.2010.11.2.173.
Autologous conditioned plasma as therapy of tendon and ligament lesions in seven horses
- Affiliations
-
- 1Equine Clinic, Faculty of Veterinary Medicine, Freie Universita Berlin, Oertzenweg 19b, 14163 Berlin, Germany.
- 2Institute for Biometrics and Data Processing, Faculty of Veterinary Medicine, Freie Universitat Berlin, Oertzenweg 19b, 14163 Berlin, Germany.
- 3Department for Large Animal Diseases, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Nowoursynowska 100, 02-786 Warsaw, Poland.
- KMID: 1110867
- DOI: http://doi.org/10.4142/jvs.2010.11.2.173
Abstract
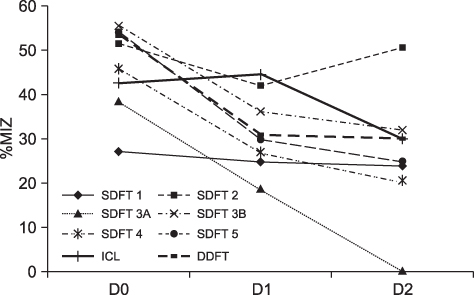
- This case report describes the intralesional application of autologous conditioned plasma (ACP) in seven horses as treatment of severe tendinitis of the superficial digital flexor tendon, deep digital flexor tendon, or desmitis of the inferior check ligament. Follow-up data of the horses revealed a positive outcome in 10 to 13 months post injury. All horses treated with ACP were either performing in their previous work-load or were back in full training. Further studies with long-term follow-up will have to be performed to support these clinical intermediate-term observations.
MeSH Terms
Figure
Reference
-
1. Appel TR, Pötzsch B, Müller J, von Lindern JJ, Bergé SJ, Reich RH. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin Oral Implants Res. 2002. 13:522–528.
Article2. Argüelles D, Carmona JU, Climent F, Muñoz E, Prades M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet Rec. 2008. 162:208–211.
Article3. Bosch G, van Schie HT, de Groot MW, Cadby JA, van de Lest CH, Barneveld A, van Weeren PR. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010. 28:211–217.
Article4. De Mos M, van der Windt AE, Jahr H, van Schie HTM, Weinans H, Verhaar JA, van Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008. 36:1171–1178.5. Dyson PK, Jackson BF, Pfeiffer DU, Price JS. Days lost from training by two- and three-year-old Thoroughbred horses: a survey of seven UK training yards. Equine Vet J. 2008. 40:650–657.
Article6. Fortier LA, Smith RK. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am Equine Pract. 2008. 24:191–201.
Article7. Künneke A, Jaugstetter H, Heyers P. Bone marrow-autologous conditioned plasma (BM-ACP) beim Pferd -erste klinische Erfahrungen einer neuen, autologen Therapieform bei der Behandlung von Sehnenerkrankungen des Pferdes. Pferdeheilkunde. 2008. 24:519–523.
Article8. Lam KH, Parkin TD, Riggs CM, Morgan KL. Descriptive analysis of retirement of Thoroughbred racehorses due to tendon injuries at the Hong Kong Jockey Club (1992-2004). Equine Vet J. 2007. 39:143–148.
Article9. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:489–496.
Article10. Perkins NR, Reid SW, Morris RS. Profiling the New Zealand Thoroughbred racing industry. 2. Conditions interfering with training and racing. N Z Vet J. 2005. 53:69–76.
Article11. Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005. 16:1043–1054.
Article12. Rantanen NW, Jorgensen JS, Genovese RL. Ross MW, Dyson SJ, editors. Ultrasonographic evaluation of the equine limb: Technique. Diagnosis and Management of Lameness in the Horse. 2003. 1st ed. Philadelphia: Saunders;166–188.
Article13. Reef VB. Superficial digital flexor tendon healing: ultrasonographic evaluation of therapies. Vet Clin North Am Equine Pract. 2001. 17:159–178.
Article14. Rossdale PD, Hopes R, Digby NJ, Offord K. Epidemiological study of wastage among racehorses 1982 and 1983. Vet Rec. 1985. 116:66–69.
Article15. Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007. 25:230–240.
Article16. Sutter WW, Kaneps AJ, Bertone AL. Comparison of hematologic values and transforming growth factor-beta and insulin-like growth factor concentrations in platelet concentrates obtained by use of buffy coat and apheresis methods from equine blood. Am J Vet Res. 2004. 65:924–930.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patellar Tendon Rupture associated with Rupture of Anterior Cruciate Ligament, Both Collateral Ligoments, and Lateral Meniscus: A Case Report
- Anatomical variations of the equine popliteal tendon
- Reconstruction of Posterior Cruciate Ligament Using Semitendinosus Tendon (3 cases)
- The Effect of Platelet Rich Plasma Dosage on the Tendon Healing in Rabbits
- Stress response as a contributing factor in horses with laminitis