Yonsei Med J.
2006 Apr;47(2):249-254. 10.3349/ymj.2006.47.2.249.
Elevated Contractile Responses to Acetylcholine in Organ Cultured Rabbit Carotid Artery
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul, Korea. dsahn@yumc.yonsei.ac.kr
- KMID: 1110750
- DOI: http://doi.org/10.3349/ymj.2006.47.2.249
Abstract
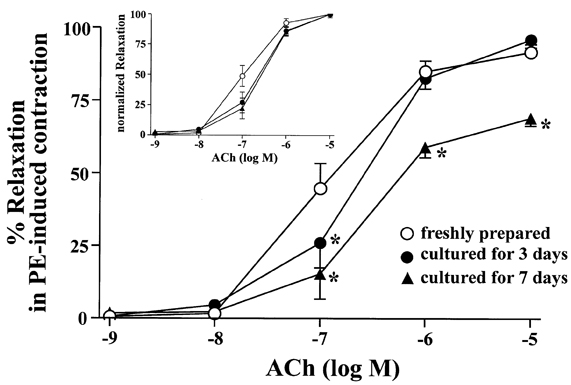
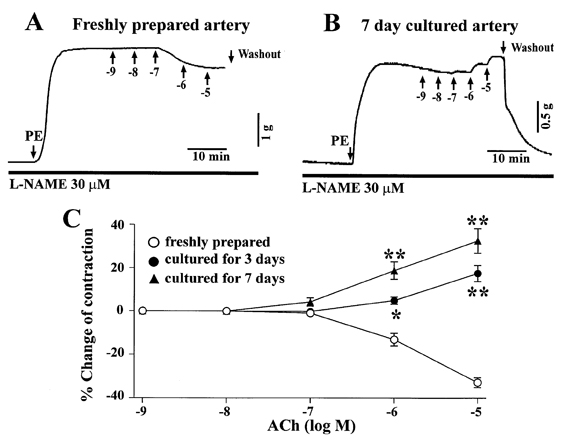
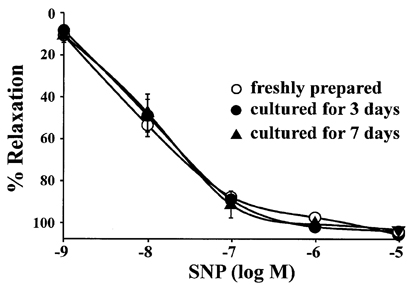
- The aim of the present study was to examine the functional changes that occur when a rabbit carotid artery is cultured in serum-free medium. In endothelium (EC)-intact arteries cultured under serum-free conditions, acetylcholine (ACh)-induced relaxation responses were partially, yet significantly, reduced when compared with freshly isolated arteries. After pretreatment with N(G)-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, application of ACh resulted in a significant contraction in organ cultured arteries. The amplitude of the ACh-induced contractions increased with the duration of culture. In EC-denuded arteries cultured under serum-free conditions, ACh induced responses similar to those in EC-intact arteries pretreated with L-NAME. Furthermore, ACh caused a significant increase in intracellular Ca2+ concentration ([Ca2+]i) in EC-denuded arteries cultured under serum-free condition for 7 days. There was little change in either [Ca2+]i or tension in freshly isolated carotid rings. There was no difference in sodium nitroprusside-induced relaxation responses between fresh and cultured arteries. These results suggest that prolonged culture of carotid arteries under serum-free conditions changes the functional properties of vascular reactivity in rabbit carotid arteries.
Keyword
MeSH Terms
-
Time Factors
Rabbits
Organ Culture Techniques/*methods
Nitroprusside/pharmacology
NG-Nitroarginine Methyl Ester/metabolism/pharmacology
*Muscle Contraction
Models, Statistical
Dose-Response Relationship, Drug
Culture Media, Serum-Free/metabolism
Carotid Arteries/*drug effects/metabolism/*pathology
Calcium/metabolism
Animals
Acetylcholine/*pharmacology
Figure
Reference
-
1. Thyberg J, Nilsson J, Palmberg L, Sjolund M. Adult human arterial smooth muscle cells in primary culture. Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985. 239:69–74.2. Chamley-Campbell JH, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979. 59:1–61.3. Davies PF. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab Invest. 1986. 55:5–24.4. Stadler E, Campbell JH, Campbell GR. Do cultured vascular smooth muscle cells resemble those of the artery wall? If not, why not? J Cardiovasc Pharmacol. 1989. 14:Suppl 6. S1–S8.5. Rogers MJ, Ward SM, Horner MA, Sanders KM, Horowitz B. Characterization of the properties of canine colonic smooth muscle in culture. Am J Physiol. 1993. 265(5 pt 1):C1433–C1442.6. Bakker ENTP, Van der Meulen ET, Spaan JAE, Vanbavel ED. Organoid culture of cannulated rat resistance arteries: effect of serum factors on vasoactivity and remodeling. Am J Physiol Heart Circ Physiol. 2000. 278:H1233–H1240.7. Lindqvist A, Nilsson BO, Hellstrand P. Inhibition of calcium entry preserves contractility of arterial smooth muscle in culture. J Vasc Res. 1997. 34:103–108.8. Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, et al. Impairment of EDR by a long-term PDGF treatment in organ-cultured rabbit mesenteric artery. Am J Physiol. 1999. 277:H318–H323.9. Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, et al. Morphological and functional changes of rabbit mesenteric artery cultured with fetal bovine serum. Life Sci. 2000. 67:807–820.10. Murata T, Suzuki N, Yamawaki H, Sato K, Hori M, Karaki H, et al. Dexamethasone prevents impairment of endothelium-dependent relaxation in arteries cultured with fetal bovine serum. Eur J Pharmacol. 2005. 515:134–141.11. Kwon SC, Park KY, Ahn DS, Lee HY, Kang BS. The effect of NO donor on contraction, cytosolic Ca2+ level and ionic currents in guinea pig ileal smooth muscle. Korean J Physiol Pharmacol. 2000. 4:33–40.12. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980. 288:373–376.13. Gryglewski RJ, Botting RM, Vane JR. Mediators produced by the endothelial cell. Hypertension. 1988. 12:530–548.14. Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983. 52:352–357.15. Karaki H, Sato K, Ozaki H, Murakami K. Effect of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988. 156:259–266.16. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990. 259(1 Pt 1):C3–C18.17. Eglen RM, Reddy H, Watson N, Challiss RA. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol Sci. 1994. 15:114–119.18. Lindqvist A, Nordstrom I, Malmqvist U, Nordenfelt P, Hellstrand P. Long-term effects of Ca2+ on structure and contractility of vascular smooth muscle. Am J Physiol. 1999. 277(1 Pt 1):C64–C73.19. Abel PW, Trapani A, Aprigliano O, Hermsmeyer K. Trophic effect of norepinephrine on rat portal vein in organ culture. Circ Res. 1980. 47:770–775.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phorbol Ester Modulates th Action of Acetylcholine in Rabbit Carotid Artery
- Contraction and Relaxation Responses of Contralateral Renal Artery in Renovascular Hypertension
- Contractile Response to Endothelin in Porcine External Carotid Arteries
- Mechanism of Acetylcholine-induced Endothelium-dependent Relaxation in the Rabbit Carotid Artery by M3-receptor Activation
- Epithelial Modulation on Guinea-pig Tracheal Smooth Muscle Tension to Contractile Agents