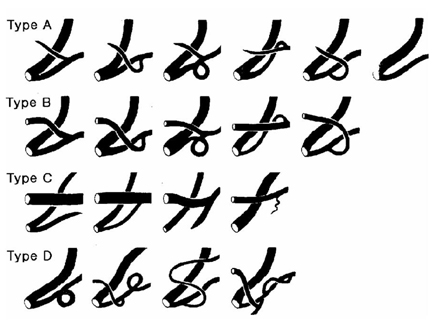Yonsei Med J.
2006 Apr;47(2):243-248. 10.3349/ymj.2006.47.2.243.
The Relationship of Anatomic Variation of Pancreatic Ductal System and Pancreaticobiliary Diseases
- Affiliations
-
- 1Division of Gastroenterology, Institute of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. jbchung@yumc.yonsei.ac.kr
- KMID: 1110749
- DOI: http://doi.org/10.3349/ymj.2006.47.2.243
Abstract
- The aims of this study were to identify the morphological diversities and anatomical variations of pancreatic ductal system and to define the relationships between pancreatic ductal systems, pancreaticobiliary diseases, and procedure-related complications, including post-ERCP pancreatitis. This study included 582 patients in whom both pancreatic duct (PD) and common bile duct were clearly visible by ERCP. PD systems were categorized into four types according to the relationship between common bile duct and PD. In types A and B, Wirsung duct formed the main PD. In type C, Wirsung duct did not form the main PD. If PD system did not fall into any of these three types, it was categorized as type D. The distribution of types among pancreatic ducts examined was as follows: type A: 491 cases (84.4%), type B: 56 cases (9.6%), type C: 20 cases (3.4%), and type D: 15 cases (2.6%). The anomalous anatomic variations of PD systems were divided into migration, fusion, and duplication anomalies. PD anomalies were noted in 51 patients, of which 19 (3.3%) were fusion anomalies (12 complete pancreas divisum, 7 incomplete pancreas divisum), and 32 (5.5%) were duplication anomalies (5 number variations, 27 form variations). No significant relationships between various PD morphologies and pancreaticobiliary diseases were found. However, post- ERCP hyperamylasemia was more frequently found in types C (41.7%), D (50%) and A (19.8%) than in type B (9.4%). In summary, whether Wirsung duct forms the main PD and the presence or absence of the opening of the Santorini duct are both important factors in determining the development of pancreatitis and hyperamylasemia after ERCP.
Keyword
MeSH Terms
-
Sex Factors
Pancreatitis/diagnosis/pathology
Pancreatic Ducts/*anatomy & histology/*pathology
Pancreatic Diseases/diagnosis
Middle Aged
Male
Humans
Female
Common Bile Duct/anatomy & histology/pathology
Cholangiopancreatography, Endoscopic Retrograde/*methods
Bile Ducts/*anatomy & histology/metabolism/pathology
Figure
Cited by 1 articles
-
Exploring the variations of the pancreatic ductal system: a systematic review and meta-analysis of observational studies
Adil Asghar, Ravi Kant Narayan, Nagavalli Basavanna Pushpa, Apurba Patra, Kumar Satish Ravi, R. Shane Tubbs
Anat Cell Biol. 2024;57(1):31-44. doi: 10.5115/acb.23.148.
Reference
-
1. Oi I, Kobayashi S, Kondo T. Endoscopic pancreatocholangiography. Endoscopy. 1970. 2:103–106.2. Griffin N, Wastle ML, Dunn WK, Ryder SD, Beckingham IJ. Magnetic resonance cholangiopancreatography versus endoscopic retrograde cholangiopancreatography in the diagnosis of choledocholithiasis. Eur J Gastroenterol Hepatol. 2003. 15:809–813.3. Talwalkar JA, Angulo P, Johnson CD, Petersen BT, Lindor KD. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology. 2004. 40:39–45.4. Hunerbein M, Stroszczynski C, Ulmer C, Handke T, Felix R, Schlag PM. Prospective comparison of transcutaneous 3-dimensional US cholangiography, magnetic resonance cholangiography, and direct cholangiography in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003. 58:853–858.5. Heiss FW, Shea JA. Association of pancreatitis and variant ductal anatomy: dominant drainage of the duct of Santorini. Am J Gastroenterol. 1978. 70:158–162.6. Phillip J, Koch H, Classen M. Variations and anomalies of the papilla of Vater, the pancreas and the biliary duct system. Endoscopy. 1974. 6:70–77.7. Belber JP, Bill K. Fusion anomalies of the pancreatic ductal system: differentiation from pathologic states. Radiology. 1977. 123:637–642.8. Jung YS, Lee KJ, Kim H, Kim WH, Kim IG, Yoo BM, et al. Risk factor for extrahepatic bile duct cancer in patients with anomalous pancreaticobiliary ductal union. Hepatogastroenterology. 2004. 51:946–949.9. Chang LY, Wang HP, Wu MS, Huang HT, Wang HH, Lin CC, et al. Anomalous pancreaticobiliary ductal union- an etiologic association of gallbladder cancer and adenomyomatosis. Hepatogastroenterology. 1998. 45:2016–2019.10. Cubilla AL, Fitzgerald PJ. Cubilla AL, Fitzgerald PJ, editors. Gross anatomy. Tumors of the exocrine pancreas. 1984. Washington, DC: Armed forces institute of pathology;31–52. 2nd series, Fascicle 19.11. Siegel HJ. Siegel HJ, editor. Radiologic interpretation: Normal biliary system and variations; normal pancreatic duct and variations. Endoscopic retrograde cholangiopancreatography: technique, diagnosis and therapy. 1992. New York: Raven Press Ltd;41–59.12. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991. 37:383–393.13. Millbourn E. On the excretory ducts of the pancreas in man, with special reference to their relations to each other, to the common bile duct and to the duodenum. A radiological and anatomical study. Acta Anat. 1950. 9:1–34.14. Dawson W, Langman J. An anatomical-radiological study on the pancreatic duct pattern in man. Anat Rec. 1961. 139:59–68.15. Kleitsch WP. Anatomy of the pancreas. Arch Surg. 1955. 71:795–802.16. Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endosc Clin N Am. 1995. 5:1–30.17. Ravitch MM, Woods AC Jr. Annular pancreas. Ann Surg. 1950. 132:1116–1127.18. Sigfusson BF, Wehlin L, Lindstrom CG. Variants of pancreatic duct system of importance in endoscopic retrograde cholangiopancreatography. Observations on autopsy specimens. Acta Radiol Diagn (Stockh). 1983. 24:113–128.19. Yatto RP, Siegel JH. Variant pancreatography. Am J Gastroenterol. 1983. 78:115–118.20. Carr-Locke DL. Pancreas divisum. the controversy goes on? Endoscopy. 1991. 23:88–90.21. Sugawa C, Walt AJ, Nunez DC, Masuyama H. Pancreas divisum: is it a normal anatomic variant? Am J Surg. 1987. 153:62–67.22. Tulassay Z, Papp J, Farkas IE. Diagnostic aspects of incomplete pancreas divisum. Gastrointest Endosc. 1986. 32:428.23. Uomo G, Manes G, D'Anna L, Laccetti M, Di Gaeta S, Rabitti PG. Fusion and duplication variants of pancreatic duct system. Clinical and pancreatographic evaluation. Int J Pancreatol. 1995. 17:23–28.24. Burtin P, Person B, Charneau J, Boyer J. Pancreas divisum and pancreatitis: a coincidental association? Endoscopy. 1991. 23:55–58.25. Delhaye M, Cremer M. Clinical significance of pancreas divisum. Acta Gastroenterol Belg. 1992. 55:306–313.26. Gregg JA. Pancreas divisum: its association with pancreatitis. Am J Surg. 1977. 134:539–543.27. Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980. 21:105–114.28. Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990. 5:248–254.29. Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998. 48:1–10.30. LaFerla G, Gordon S, Archibald M, Murray WR. Hyperamylasaemia and acute pancreatitis following endoscopic retrograde cholangiopancreatography. Pancreas. 1986. 1:160–163.31. Johnson GK, Geenen JE, Johanson JF, Sherman S, Hogan WJ, Cass O. Evaluation of post-ERCP pancreatitis: potential causes noted during controlled study of differing contrast media. Midwest Pancreaticobiliary Study Group. Gastrointest Endosc. 1997. 46:217–222.32. Christoforidis E, Goulimaris I, Kanellos I, Tsalis K, Demetriades C, Betsis D. Post-ERCP pancreatitis and hyperamylasemia: patient-related and operative risk factors. Endoscopy. 2002. 34:286–292.33. Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996. 335:909–918.34. Dickinson RJ, Davies S. Post-ERCP pancreatitis and hyperamylasaemia: the role of operative and patient factors. Eur J Gastroenterol Hepatol. 1998. 10:423–428.35. Tulassay Z, Papp J, Koranyi L, Szathmari M, Tamas G Jr. Hormonal and biochemical changes following endoscopic retrograde cholangio-pancreatography. Acta Gastroenterol Belg. 1981. 44:538–544.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anatomical Variations and Morphological Diversities of the Pancreatic Ductal System: Clinical and ERCP evaluation
- The Pancreaticobiliary Ductal Union System and Associated Disorders
- A Case of Choledochal Cyst (Type IVa) and Anomalous Pancreaticobiliary Ductal Union Combined with Pancreatic Duct Stone
- Analysis of 45 Cases of Anomalous Pancreaticobiliary Ductal Union
- A Pancreaticobiliary Maljunction (New Komi Classification, Type IIIc3) with a Choledochal Cyst, Common Bile Duct Stones and Pancreatic Duct Stones