Yonsei Med J.
2011 Jan;52(1):151-157. 10.3349/ymj.2011.52.1.151.
Effect of Intravitreal Bevacizumab on Vascular Endothelial Growth Factor Expression in Patients with Proliferative Diabetic Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, NHIC Ilsan Hospital, Goyang, Korea.
- 2Department of Ophthalmology, Emory Eye Center, Emory University School of Medicine, Atlanta, Georgia, USA.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Pathology, NHIC Ilsan Hospital, Goyang, Korea.
- 5Department of Pathology, Emory Eye Center, Emory University School of Medicine, Atlanta, Georgia, USA.
- 6The Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. hjkoh@yuhs.ac
- KMID: 1106450
- DOI: http://doi.org/10.3349/ymj.2011.52.1.151
Abstract
- PURPOSE
To investigate the effect of bevacizumab (Avastin; Genentech, San Francisco, CA, USA) on vascular endothelial growth factor (VEGF) expression and inflammation in fibrovascular membranes in patients with proliferative diabetic retinopathy (PDR).
MATERIALS AND METHODS
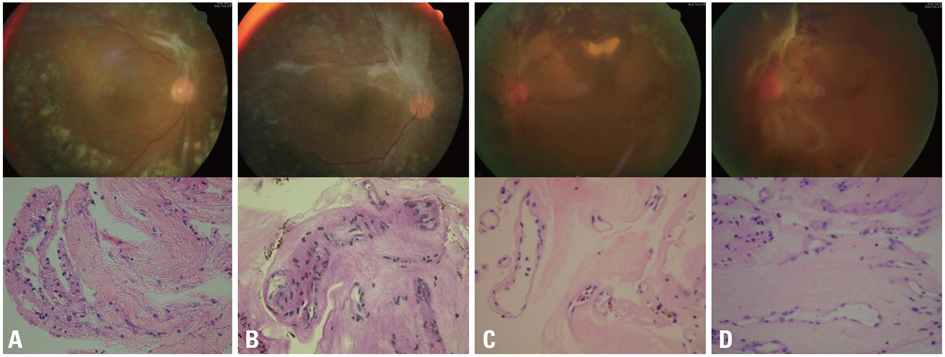
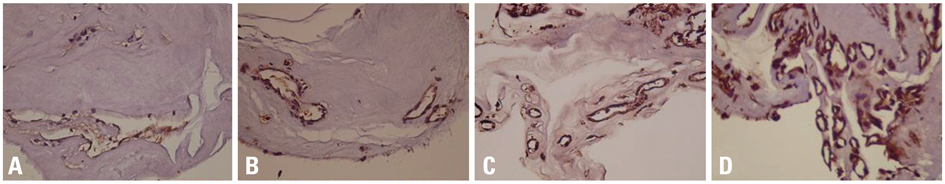
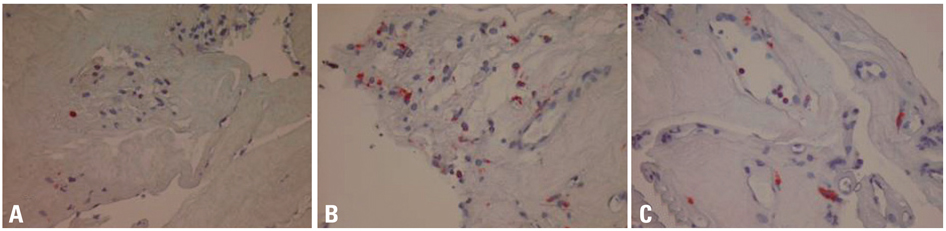
Fibrovascular membranes from 19 eyes of 18 patients with PDR were studied using immunohistochemistry and analyzed in the following 3 groups; group 1: 4 inactive PDR eyes, group 2: 10 active PDR eyes treated preoperatively with adjunctive intravitreal bevacizumab, group 3: five active PDR eyes not treated preoperatively with bevacizumab. Immunohistochemical staining for VEGF, CD31 and CD68 were done.
RESULTS
The immunoreactivity to VEGF and CD 31-positive blood vessels was significantly higher in membranes from group 3 than group 1 (p = 0.007 for VEGF, 0.013 for CD 31-positive vessels). Intravitreal bevacizumab caused a reduction in VEGF expression and vascular densities in 4 out of 10 (40%) excised membranes from eyes with PDR. However, six membranes (60%) in group 2 still demonstrated relatively strong VEGF expression and high vascular density. Infiltration of macrophages was observed in 16 out of the 19 membranes, and the density of macrophages was increased in group 2 compared with group 1 (p = 0.043).
CONCLUSION
Intravitreal bevacizumab injections caused some reduction in VEGF expression and vascular densities in a limited number of active PDR patients. A single intravitreal bevacizumab injection may not be enough to induce complete blockage of VEGF and pathologic neovascularization in active PDR patients. Repeated injections, panretinal photocoagulation and/or PPV may be necessary following intravitreal bevacizumab to reinforce the anti-VEGF effect of the drug.
Keyword
MeSH Terms
Figure
Reference
-
1. Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994. 118:445–450.
Article2. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.
Article3. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998. 82:561–568.
Article4. Chen YS, Hackett SF, Schoenfeld CL, Vinores MA, Vinores SA, Campochiaro PA. Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br J Ophthalmol. 1997. 81:919–926.
Article5. Matsuoka M, Ogata N, Minamino K, Matsumura M. Expression of pigment epithelium-derived factor and vascular endothelial growth factor in fibrovascular membranes from patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2006. 50:116–120.6. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005. 25:111–118.7. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.
Article8. Bhavsar AR. Diabetic retinopathy: the latest in current management. Retina. 2006. 26:S71–S79.
Article9. Meleth AD, Agrón E, Chan CC, Reed GF, Arora K, Byrnes G, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005. 46:4295–4301.
Article10. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005. 115:1111–1119.
Article11. Minnella AM, Savastano CM, Ziccardi L, Scupola A, Falsini B, Balestrazzi E. Intravitreal bevacizumab (Avastin) in proliferative diabetic retinopathy. Acta Ophthalmol. 2008. 86:683–687.
Article12. Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008. 246:1699–1705.
Article13. Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113:1695.e1–1695.e15.
Article14. Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009. 116:927–938.
Article15. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006. 26:699–700.
Article16. Arimura N, Otsuka H, Yamakiri K, Sonoda Y, Nakao S, Noda Y, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology. 2009. 116:921–926.
Article17. Matsuyama K, Ogata N, Jo N, Shima C, Matsuoka M, Matsumura M. Levels of vascular endothelial growth factor and pigment epithelium-derived factor in eyes before and after intravitreal injection of bevacizumab. Jpn J Ophthalmol. 2009. 53:243–248.
Article18. Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007. 125:1363–1366.
Article19. Kubota T, Aoki R, Harada Y, Tou N, Kohno Y, Tawara A, et al. Trabecular meshwork in neovascular glaucoma eyes after the intravitreal injection of bevacizumab. Br J Ophthalmol. 2009. 93:557–558.
Article20. Kubota T, Morita H, Tou N, Nitta N, Tawara A, Satoh H, et al. Histology of fibrovascular membranes of proliferative diabetic retinopathy after intravitreal injection of bevacizumab. Retina. 2010. 30:468–472.
Article21. Arevalo JF, Wu L, Sanchez JG, Maia M, Saravia MJ, Fernandez CF, et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye (Lond). 2009. 23:117–123.
Article22. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996. 87:3336–3343.
Article23. Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990. 172:1535–1545.
Article24. Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002. 160:501–509.
Article25. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001. 276:7614–7620.
Article26. Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S, et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998. 82:845–851.
Article27. Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994. 55:410–422.
Article28. Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995. 1:1024–1028.
Article29. Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006. 290:H547–H559.
Article30. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006. 290:H560–H576.
Article31. Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999. 126:2141–2147.
Article32. Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997. 124:3633–3638.
Article33. Lang RA. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ. 1997. 4:12–20.
Article34. Tatar O, Yoeruek E, Szurman P, Bartz-Schmidt KU, Adam A, et al. Tübingen Bevacizumab Study Group. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008. 126:782–790.
Article35. Chen E, Hsu J, Park CH. Acute visual acuity loss following intravitreal bevacizumab for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2009. 40:68–70.
Article36. Shimura M, Yasuda K. Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br J Ophthalmol. 2010. 94:381–383.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-term Effect of Intravitreal Bevacizumab Injection Preventing Panretinal Photocoagulation-Induced Macular Edema in Proliferative Diabetic Retinopathy
- Retinal Vascular Caliber Changes in Diabetic Retinopathy after Panretinal Photocoagulation and Additive Bevacizumab Injections
- The Study of Intraocular Vascular Endothelial Growth Factor Concentration in Diabetic Retinopathy after Panretinal Photocoagulation
- Effects of an Intravitreal Bevacizumab Injection Combined With Panretinal Photocoagulation on High-Risk Proliferative Diabetic Retinopathy
- A Case of Rapid Progression to Proliferative Diabetic Retinopathy Associated with Generalized Edema