J Vet Sci.
2008 Jun;9(2):183-191. 10.4142/jvs.2008.9.2.183.
Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat
- Affiliations
-
- 1Department of Veterinary Surgery and Radiology, West Bengal University of Animal and Fishery Sciences, Kolkata, India.
- 2Bioceramics and Coating Division, Central Glass and Ceramic Research Institute, Kolkata, India. biswa_kundu@rediffmail.com
- KMID: 1106234
- DOI: http://doi.org/10.4142/jvs.2008.9.2.183
Abstract
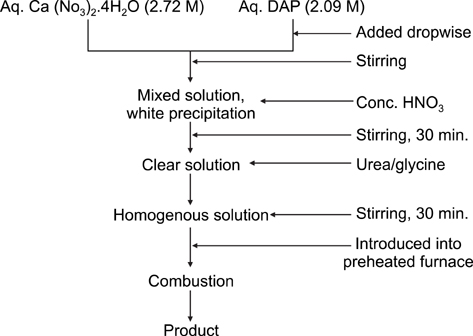
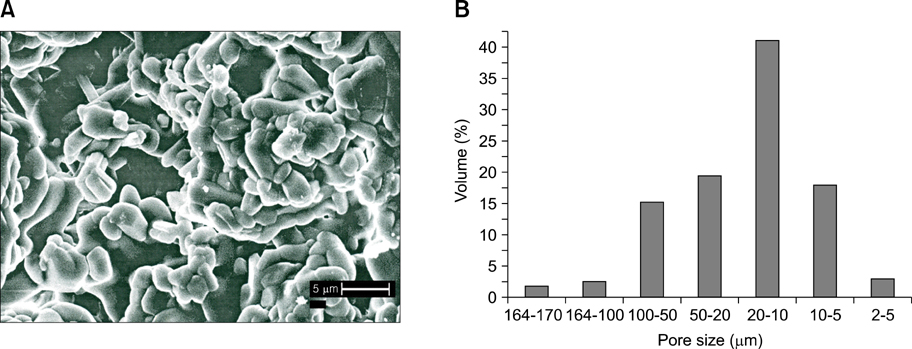
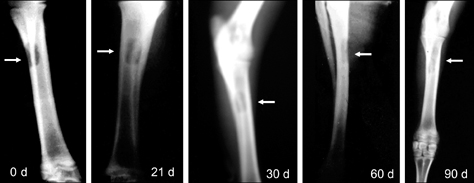
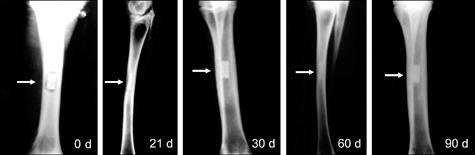
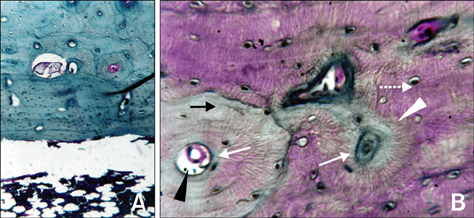
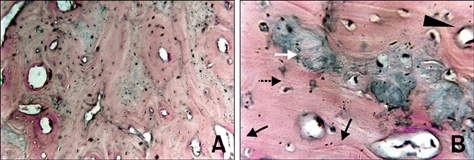
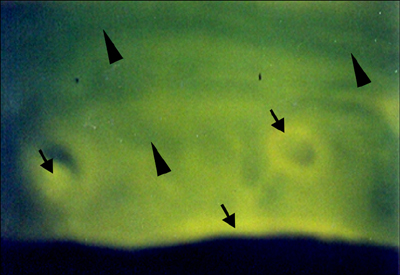
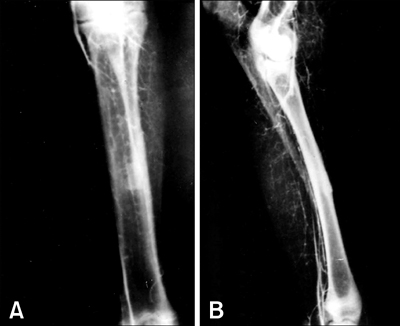
- The present study was undertaken to evaluate porous hydroxyapatite (HAp), the powder of which was prepared by a novel aqueous solution combustion technique, as a bone substitute in healing bone defects in vivo, as assessed by radiologic and histopathologic methods, oxytetracycline labeling, and angiogenic features in Bengal goat. Bone defects were created in the diaphysis of the radius and either not filled (group I) or filled with a HAp strut (group II). The radiologic study in group II showed the presence of unabsorbed implants which acted as a scaffold for new bone growth across the defect, and the quality of healing of the bone defect was almost indistinguishable from the control group, in which the defect was more or less similar, although the newly formed bony tissue was more organized when HAp was used. Histologic methods showed complete normal ossification with development of Haversian canals and well-defined osteoblasts at the periphery in group II, whereas the control group had moderate fibro-collagenization and an adequate amount of marrow material, fat cells, and blood vessels. An oxytetracycline labeling study showed moderate activity of new bone formation with crossing-over of new bone trabeculae along with the presence of resorption cavities in group II, whereas in the control group, the process of new bone formation was active from both ends and the defect site appeared as a homogenous non-fluoroscent area. Angiograms of the animals in the control group showed uniform angiogenesis in the defect site with establishment of trans-transplant angiogenesis, whereas in group II there was complete trans-transplant shunting of blood vessel communication. Porous HAp ceramic prepared by an aqueous combustion technique promoted bone formation over the defect, confirming their biologic osteoconductive property.
Keyword
MeSH Terms
Figure
Reference
-
1. Alexander H, Parsons JR, Ricci J, Bajpai PK. Calcium-based ceramics and composites in bone reconstruction. CRC Crit Rev Biocompat. 1987. 4:43–77.2. Asada M, Oukami K, Nakamura S, Takahashi K. Effect of powder characteristics on the sinterability calcium hydroxyapatite. J Ceram Soc Japan. 1987. 95:703–709.
Article3. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995. 20:1055–1060.
Article4. Bolander ME, Balian G. The use of demineralized bone matrix in the repair of segmental defects. Augmentation with extracted matrix proteins and a comparison with autologous grafts. J Bone Joint Surg Am. 1986. 68:1264–1274.
Article5. Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999. 30:571–581.6. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998. 16:155–162.
Article7. Cavadias AX, Trueta J. An experimental study of the vascular contribution to the callus of fracture. Surg Gynecol Obstet. 1965. 120:731–747.8. Chapman MW. Chapman MW, Madison M, editors. Bone grafting. Operative Orthopaedics. 1993. Philadelphia: JB Lippincott;139–149.9. Cowley SP, Anderson LD. Hernias through donor sites for iliac-bone grafts. J Bone Joint Surg Am. 1983. 65:1023–1025.
Article10. Daculsi G, Hartmann DJ, Heughebaert M, Hamel L, Le Nihouannen JC. In vivo cell interactions with calcium phosphate bioceramics. J Submicrosc Cytol Pathol. 1988. 20:379–384.11. Ferraro JW. Experimental evaluation of ceramic calcium phosphate as a substitute for bone grafts. Plast Reconstr Surg. 1979. 63:634–640.
Article12. Ghosh SK, Datta S, Roy SK. Solution combustion synthesis of calcium hydroxyapatite nanoparticles. Trans Ind Ceram Soc. 2004. 63:27–32.
Article13. Gibson CJ, Thornton VF, Brown WA. Incorporation of tetracycline into impeded and unimpeded mandibular incisors of the mouse. Calcif Tissue Res. 1978. 26:29–31.
Article14. Gil-Albarova J, Garrido-Lahiguera R, Salinas AJ, Román J, Bueno-Lozano AL, Gil-Albarova R, Vallet-Regí MM. The in vivo performance of a sol-gel glass and a glass-ceramic in the treatment of limited bone defects. Biomaterials. 2004. 25:4639–4645.
Article15. Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987. 225:7–16.
Article16. Hattori T, Iwadate Y, Kato T. Hydrothermal synthesis of hydroxyapatite from calcium acetate and triethyl phosphate. Adv Ceram Mater. 1988. 3:426–428.
Article17. Hench LL, Ethridge EC. Biomaterials. An Interfacial Approach. 1982. New York: Academic Press.18. Holmes RE, Buchloz RW, Mooney V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study. J Bone Joint Surg Am. 1986. 68:904–911.
Article19. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981. 157:259–278.
Article20. Kazuo A, Fumiaki M, Kojiro K, Katsuyuki F. Clinical application of pure β-TCP for bone tumors. J Joint Surg. 2002. 21:1501–1506.21. König B Jr, Beck TJ, Kappert HF, Kappert CC, Masuko TS. A study of different calcification areas in newly formed bone 8 weeks after insertion of dental implants in rabbit tibias. Ann Anat. 1998. 180:471–475.
Article22. Lane JM, Tomin E, Bostrom MP. Biosynthetic bone grafting. Clin Orthop Relat Res. 1999. 367:Suppl. S107–S117.
Article23. LeGeros RZ, Daculsi G. Yamamuro T, Hench LL, Wilson J, editors. In vivo transformation of bisphasic calcium phosphate ceramics: Ultrastructural and physicochemical characterizations. CRC Handbook of Bioactive Ceramics. 1990. Boca Raton: CRC Press;17–28.24. LeGeros RZ, Parsons JR, Daculsi G, Driessens F, Lee D, Liu ST, Metsger S, Peterson D, Walker M. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation-bioresorption. Ann NY Acad Sci. 1988. 523:268–271.
Article25. Maiti SK, Singh GR. Different types of bone-grafts and ceramic implants in goats - A triple fluorochrome labeling study. Indian J Anim Sci. 1995. 65:140–143.26. Maiti SK, Singh GR. Vascularization of composite bone grafts and ceramic implants in goats. Small Rumin Res. 1996. 20:171–176.
Article27. Parasnalli B. A comparative study on the osteoinductive property, healing process and fate of decalcified autoclaved, deep frozen and organic allogenic cortical bone grafts in bovines. 1988. Izatnagar, India: Indian Veterinary Research Institute;MV Sc Thesis.28. Park JB. Biomaterials Science and Engineering. 1987. New York: Plenum Press.29. Parsons JR, Ricci JL, Alexander H, Bajpai PK. Osteoconductive composite grouts for orthopedic use. Ann N Y Acad Sci. 1988. 523:190–207.
Article30. Shimazaki K, Mooney V. Comparative study of porous hydroxyapatite and tricalcium phosphate as bone substitute. J Orthop Res. 1985. 3:301–310.
Article31. Shors EC. Coralline bone graft substitutes. Orthop Clin North Am. 1999. 30:599–613.
Article32. Shukla BP. A comparative evaluation of fresh autogenous vis-à-vis freeze dried and decalcified freeze dried segmental xenogenous bone grafts in dogs. 1989. Izatnagar, India: Indian Veterinary Research Institute;MV Sc Thesis.33. Simmons DJ. Urist MR, editor. Fracture healing. Fundamentals and Clinical Bone Physiology. 1980. Philadelphia: Lippincott;331–368.34. Singh H. Surgical studies on grafting materials for better osteogenesis in animals. Indian Council of Agricultural Research Final Report. 1978. Pantnagar, India:35. Singh S. Reconstruction of segmental ulnar defect with tricalcium phosphate, calcium hydroxyapatite and calcium hydroxyapatite-bone matrix combination in rabbit. 1998. Izatnagar, India: Indian Veterinary Research Institute;MV Sc Thesis.36. Summers BN, Eisenstein SM. Donor site pain from the ileum. A complication of lumbar spine fusion. J Bone Joint Surg. 1989. 71:677–680.37. Suryawanshi SB, Maiti SK, Singh GR. Fluorochrome labelling studies on the effect of anabolic hormones in fracture healing in dogs. Indian J Vet Surg. 1999. 20:28–30.38. Tomford WW, Mankin HJ. Bone banking. Update on methods and materials. Orthop Clin North Am. 1999. 30:565–570.39. Uchida A, Nade SML, McCartney ER, Ching W. The use of ceramics for bone replacement. A comparative study of three different porous ceramics. J Bone Joint Surg Br. 1984. 66:269–275.
Article40. Vacanti CA, Bonassar LJ. An overview of tissue engineered bone. Clin Orthop Relat Res. 1999. 367:Suppl. S375–S381.
Article41. Varshney AC, Singh H, Kumar A. Angiographic studies on experimental osteomyelitis in dog. Indian J Vet Surg. 1994. 15:74–77.42. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989. 3:192–195.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Silk Fibroin/Nano-hydroxyapatite/Corn Starch Composite Porous Scaffold on Bone Regeneration in the Rabbit Calvarial Defect Model
- The effects of Hydroxyapatite nano-coating implants on healing of surgically created circumferential gap in dogs
- A Comparative Study on Regeneration of Bone Defects after the Grafts of Demineralized Bone Matrix and Hydroxyapatite
- Effects of Topically Applied 0.1%Dexamethasone on Endothelial Healing and Aqueous Composition Following Experimental Corneal Alkali Wounds
- Wettability and drug delivery of functionally graded nano-micro porous titanium surface