J Vet Sci.
2010 Mar;11(1):67-72. 10.4142/jvs.2010.11.1.67.
Detection of Streptococcus dysgalactiae subsp. equisimilis in equine nasopharyngeal swabs by PCR
- Affiliations
-
- 1Department of Veterinary Science, University of Camerino, Via Circonvallazione 93/95, 62024 Matelica, Italy. silvia.preziuso@unicam.it
- 2Department of Technology and Biotechnology of Animal Productions, Infectious Diseases Unit, University of Perugia, Via S. Costanzo 4, 06126 Perugia, Italy.
- KMID: 1106159
- DOI: http://doi.org/10.4142/jvs.2010.11.1.67
Abstract
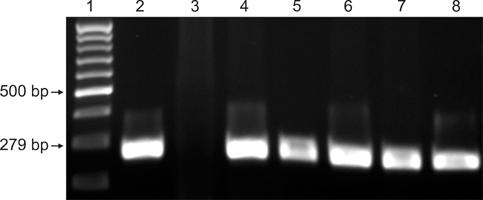
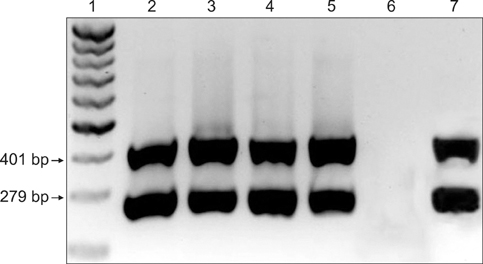
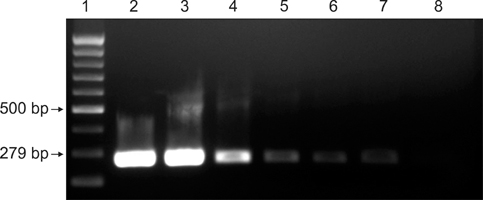
- Streptococcus (S.) dysgalactiae subsp. equisimilis is responsible for severe diseases in humans, including primary bacteraemia, pneumonia, endocarditis, and toxic shock syndrome. Infection in some animal species can also occur, although a few studies have looked into cross-species infectivity. In horses, S. equisimilis is generally considered infrequent or opportunistic, but has recently been isolated from cases of strangles-like disease. Rapid and sensitive diagnostic techniques could enable epidemiological studies and effective investigation of outbreaks involving these bacteria. In this study, PCR protocols previously described in cattle and in humans to detect the species S. dysgalactiae and the subspecies equisimilis were evaluated to detect specific sequences in equine samples. For this purpose, 99 monolateral nasal swabs were collected from horses from stud farms with a history of S. equisimilis infection and were tested blindly by bacteriological isolation and by single and duplex PCR. DNA for PCR was extracted both from the colonies grown on agar media and from enrichment broth aliquots after incubation with nasal swab samples. S. equisimilis was identified by bacteriological isolation in 23 out of 99 swab samples, and PCR assays on these colonies were fully concordant with bacteriological identification (kappa statistic = 1.00). In addition, PCR of the enrichment broth aliquots confirmed the bacteriological results and detected S. equisimilis in 6 samples more than the bacteriological examination (kappa statistic = 0.84). The PCR protocols appeared to be reliable for the rapid identification of S. equisimilis in equine nasal swab samples, and could be useful for microbiological diagnosis.
Keyword
MeSH Terms
-
Animals
NA, Bacterial/chemistry/genetics
Horse Diseases/diagnosis/*microbiology
Horses
Limit of Detection
Male
Nasopharynx/microbiology
Polymerase Chain Reaction/methods/*veterinary
Respiratory Tract Infections/diagnosis/microbiology/*veterinary
Sensitivity and Specificity
Streptococcal Infections/diagnosis/microbiology/*veterinary
Streptococcus/genetics/*isolation & purification
Figure
Reference
-
1. Alber J, El-Sayed A, Lämmler C, Hassan AA, Weiss R, Zschöck M. Multiplex Polymerase Chain Reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. J Vet Med B Infect Dis Vet Public Health. 2004. 51:455–458.
Article2. Berenguer J, Sampedro I, Cercenado E, Baraia J, Rodríguez-Créixems M, Bouza E. Group-C beta-hemolytic streptococcal bacteremia. Diagn Microbiol Infect Dis. 1992. 15:151–155.3. Blanchard PC, Fiser KM. Streptococcus dysgalactiae polyarthritis in dairy goats. J Am Vet Med Assoc. 1994. 205:739–741.4. Caballero AR, Lottenberg R, Johnston KH. Cloning, expression, sequence analysis, and characterization of streptokinases secreted by porcine and equine isolates of Streptococcus equisimilis. Infect Immun. 1999. 67:6478–6486.
Article5. Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, Hasegawa T, Ohta M. Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. 2004. 42:186–192.
Article6. Hong CB, Donahue JM, Giles RC Jr, Petrites-Murphy MB, Poonacha KB, Roberts AW, Smith BJ, Tramontin RR, Tuttle PA, Swerczek TW. Etiology and pathology of equine placentitis. J Vet Diagn Invest. 1993. 5:56–63.
Article7. Katsumi M, Kataoka Y, Takahashi T, Kikuchi N, Hiramune T. Bacterial isolation from slaughtered pigs associated with endocarditis, especially the isolation of Streptococcus suis. J Vet Med Sci. 1997. 59:75–78.
Article8. Kawata K, Minakami T, Mori Y, Katsumi M, Kataoka Y, Ezawa A, Kikuchi N, Takahashi T. rDNA sequence analyses of Streptococcus dysgalactiae subsp. equisimilis isolates from pigs. Int J Syst Evol Microbiol. 2003. 53:1941–1946.
Article9. Kumar A, Sandoe J, Kumar N. Three cases of vertebral osteomyelitis caused by Streptococcus dysgalactiae subsp. equisimilis. J Med Microbiol. 2005. 54:1103–1105.
Article10. Laus F, Preziuso S, Spaterna A, Beribè F, Tesei B, Cuteri V. Clinical and epidemiological investigation of chronic upper respiratory diseases caused by Beta-haemolytic Streptococci in horses. Comp Immunol Microbiol Infect Dis. 2007. 30:247–260.
Article11. McGinn T, Wyer PC, Newman TB, Keitz S, Leipzig R, Guyatt G. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ. 2004. 171:1369–1373.
Article12. Nakamura M, Honda K, Tun Z, Ogura Y, Matoba R. Application of in situ PCR to diagnose pneumonia in medico-legal autopsy cases. Leg Med. 2001. 3:127–133.
Article13. Nomoto R, Munasinghe LI, Jin DH, Shimahara Y, Yasuda H, Nakamura A, Misawa N, Itami T, Yoshida T. Lancefield group C Streptococcus dysgalactiae infection responsible for fish mortalities in Japan. J Fish Dis. 2004. 27:679–686.
Article14. Ortel TL, Kallianos J, Gallis HA. Group C streptococcal arthritis: case report and review. Rev Infect Dis. 1990. 12:829–837.
Article15. Riffon R, Sayasith K, Khalil H, Dubreuil P, Drolet M, Lagacé J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J Clin Microbiol. 2001. 39:2584–2589.
Article16. Timoney JF. The pathogenic equine streptococci. Vet Res. 2004. 35:397–409.
Article17. Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996. 46:774–781.
Article18. Vieira VV, Teixeira LM, Zahner V, Momen H, Facklam RR, Steigerwalt AG, Brenner DJ, Castro ACD. Genetic relationships among the different phenotypes of Streptococcus dysgalactiae strains. Int J Syst Bacteriol. 1998. 48:1231–1243.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Neonatal Meningitis Caused by Streptococcus dysgalactiae subspecies dysgalactiae and Herpes Simplex Virus
- Identification of streptococcus dysgalactiae subsp. equisimilis from septic knee by 16S rRNA gene sequencing
- Streptococcus dysgalactiae subsp. equisimilis Possessing Lancefield's Group A Antigen
- Pathologic and molecular characterization of Streptococcus dysgalactiae subsp. equisimilis infection in neonatal piglets
- Comparison of Characteristics of Streptococcus dysgalactiae subsp. equisimilis Isolates Causing Repetitive vs Single Infections