J Vet Sci.
2006 Mar;7(1):1-11. 10.4142/jvs.2006.7.1.1.
BSE situation and establishment of Food Safety Commission in Japan
- Affiliations
-
- 1Department of Molecular Immunology, Graduate School of Agricultural and Life Sciences, University of Tokyo, Yayoi 1-1-1, Tokyo 113-8657, Japan. aonoder@mail.ecc.u-tokyo.ac.jp
- KMID: 1103546
- DOI: http://doi.org/10.4142/jvs.2006.7.1.1
Abstract
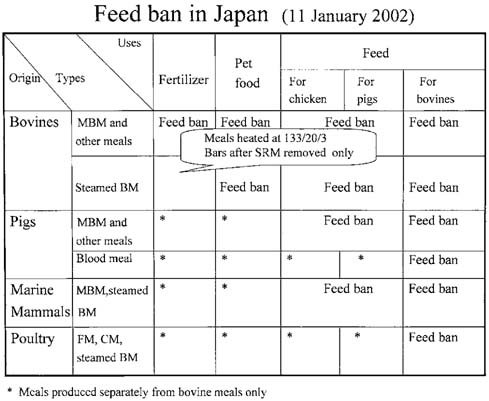
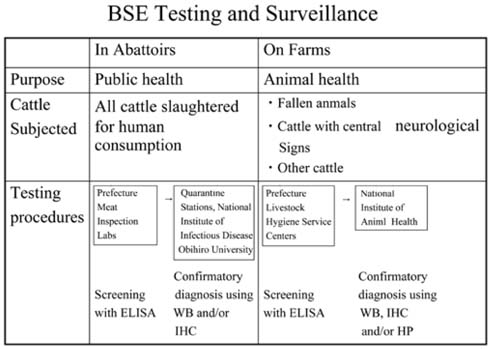
- Eight major policies were implemented by Japanese Government since Oct. 2001, to deal with bovine spongiform encephalopathy (BSE). These are; 1) Surveillance in farm by veterinarian, 2) Prion test at healthy 1.3mi cows/yr, by veterinarian, 3) Elimination of specified risk material (SRM), 4) Ban of MBM for production, sale use, 5) Prion test for fallen stocks, 6) Transparent information and traceability, 7) New Measures such as Food Safety Basic Law, and 8) Establish of Food Safety Commission in the Cabinet Office. At this moment, the extent of SRM risk has only been indicated by several reports employing tests with a limited sensitivity. There is still a possibility that the items in the SRM list will increase in the future, and this indiscriminately applies to Japanese cattle as well. Although current practices of SRM elimination partially guarantee total food safety, additional latent problems and imminent issues remain as potential headaches to be addressed. If the index of SRM elimination cannot guarantee reliable food safety, we have but to resort to total elimination of tissues from high risk-bearing and BSE-infected animals. However, current BSE tests have their limitations and can not yet completely detect highrisk and/or infected animals. Under such circumstances, tissues/wastes and remains of diseased, affected fallen stocks and cohort animals have to be eliminated to prevent BSE invading the human food chain systems. The failure to detect any cohort should never be allowed to occur, and with regular and persistent updating of available stringent records, we are at least adopting the correct and useful approach as a reawakening strategy to securing food safety. In this perspective, traceability based on a National Identification System is required.
Keyword
MeSH Terms
Figure
Reference
-
1. Baker HF, Ridley RM, Wells GAH. Experimental transmission of BSE and scrapie to the common marmoset. Vet Rec. 1993. 132:403–406.
Article2. Brown DA, Bruce M, Fraser JR. Comparison of the neuropathological characteristics of bovine spongiform encephalopathy (BSE) and variant Creutzfeldt-Jakob disease (vCJD) in mice. Neuropathol Appl Neurobiol. 2003. 29:262–272.
Article3. Brown P, Liberski PP, Wolff A, Gajdusek DC. Resistance of scrapie to steam autoclaving after formaldehyde fixation and limited survival after ashing at 360℃: pactical and theoretical implications. J Infect Dis. 1990. 161:467–472.
Article4. Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Tansmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci. 1994. 343:405–411.5. Bruce M, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmission to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature. 1997. 389:498–501.
Article6. Collinge J, Palmer MS, Sidle KCL, Hill AF, Gowland I, Meads J, Asante E, Bradley R, Doey LJ, Lantos PL. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature. 1995. 378:779–783.
Article7. Cunningham AA, Wells GAH, Scott AC, Kirkwood JK, Barnett JEF. Transmissible spongiform encephalopathy in greater kudu (Tragelaphus strepsiceros). Vet Rec. 1993. 132:68.
Article8. Di Martino A, Safar J, Gibbs CJ. The consistent use of organic solvents for purification of phospholipids from brain tissue effectively removes scrapie infectivity. Biologicals. 1994. 22:221–225.
Article9. Hoinville LJ, Wilesmith JW, Richards MS. An investigation of risk-factors for case of bovine spongiform encephalopathy born after the introduction of the feed ban. Vet Rec. 1995. 136:312–318.
Article10. Hope J, Rekkie LTD, Hunter N, Multhaup G, Beyreuther K, White H, Scott AC, Stack MJ, Dawson M, Wells GAH. Fibrils from brains of cows with new cattle disease contain scrapie-associated protein. Nature. 1988. 336:390–392.
Article11. Kirkwood JK, Wells GAH, Wilesmith JW, Cunningham AA, Jackson SI. Spongiform encephalopathy in an Arabian oryx (Oryx leucoryx) and a greater kudu (Tragelaphus strepsiceros). Vet Rec. 1990. 127:418–420.12. Kirkwood JK, Wells GAH, Cunningham AA, Jackson SI, Scott AC, Dawson M, Wilesmith JW. Scrapie-like encephalopathy in a greater kudu (Tragelaphus strepsiceros) which had not been fed ruminant-derived protein. Vet Rec. 1992. 130:365–367.
Article13. Kirkwood JK, Cunningham AA. Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Vet Rec. 1994. 135:296–303.
Article14. Onodera T, Saeki K. Japanese scrapie cases. Jpn J Infect Dis. 2000. 53:56–61.15. Peet RL, Curran JM. Spongiform encephalopathy in an imported cheetah (Acinonyx jubatus). Aust Vet J. 1992. 69:171.16. Scientific Steering Committee. Opinion on the Scientific Steering Committee on the human exposure risk (HER) via food with respect to BSE. 10th December, 1999.17. Scientific Steering Committee. Opinion on TSE infectivity distribution in ruminant tissues. 10-11th January, 2001.18. Taylor DM. Scrapie agent decontamination: implications for bovine spongiform encephalopathy. Vet Rec. 1989. 124:291–292.
Article19. Taylor DM, Fraser H, McConnell I, Brown DA, Lamza KA, Smith GRA. Decontamination studies with the agents of bovine spongiform encephalopathy and scrapie. Arch Virol. 1994. 139:313–326.
Article20. Wells G AH, Scott AC, Johnson CT, Gunnings RF, Hanock RD, Jeffrey M, Dawson M, Bradley R. A novel progressive encephalopathy in cattle. Vet Rec. 1987. 121:419–420.21. Wijeratne W VS, Curnow RN. A study of the inheritance of susceptibility of bovine spongiform encephalopathy. Vet Rec. 1990. 126:5–8.22. Wilesmith JW, Ryan JBM, Atkinson MJ. Bovine spongiform encephalopathy: epidemiological studies on the origin. Vet Rec. 1991. 128:199–203.
Article23. Wilesmith JW, Ryan JBM. Bovine spongiform encephalopathy: recent observations on the age-specific incidences. Vet Rec. 1992. 130:491–492.
Article24. Wilesmith JW. Bovine spongiform encephalopathy: epidemiological factors associated with the emergence of an important new animal pathogen in Great Britain. Semin Virol. 1994. 5:179–187.
Article25. Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996. 347:921–925.
Article26. Willoughby K, Kelly DF, Lyon DG, Wells GAH. Spongiform encephalopathy in a captive puma (Felis concolor). Vet Rec. 1992. 131:431–434.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the BSE Educational Program on BSE Practice: Based on the Individually Prescribed Instruction Model
- A Study on BSE - related Knowledge, Attitude, and Practice of Junior Nursing College Students
- The Difference of Women's Knowledge, Attitudes and Practice Education after Education for Breast Self-examination
- Differences in Health Belief by Compliance Level with Breast Self-Examination and Predictors of BSE among Women
- Knowledge, Attitude, and Practice of Obstetric Nurses in Relation to Breast Cancer and Breast Self-examination