Korean J Radiol.
2011 Dec;12(6):731-739. 10.3348/kjr.2011.12.6.731.
Pancreatic Tumors: Emphasis on CT Findings and Pathologic Classification
- Affiliations
-
- 1Department of Radiology and the Research Institute of Radiological Sciences, Severance Hospital, Yonsei University College of Medicine, Seoul 120-752, Korea. gafield2@yuhs.ac
- KMID: 1101928
- DOI: http://doi.org/10.3348/kjr.2011.12.6.731
Abstract
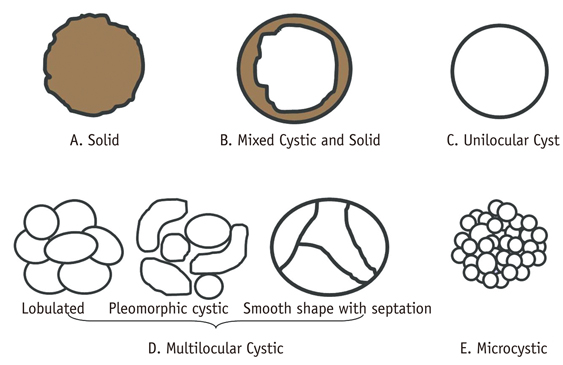
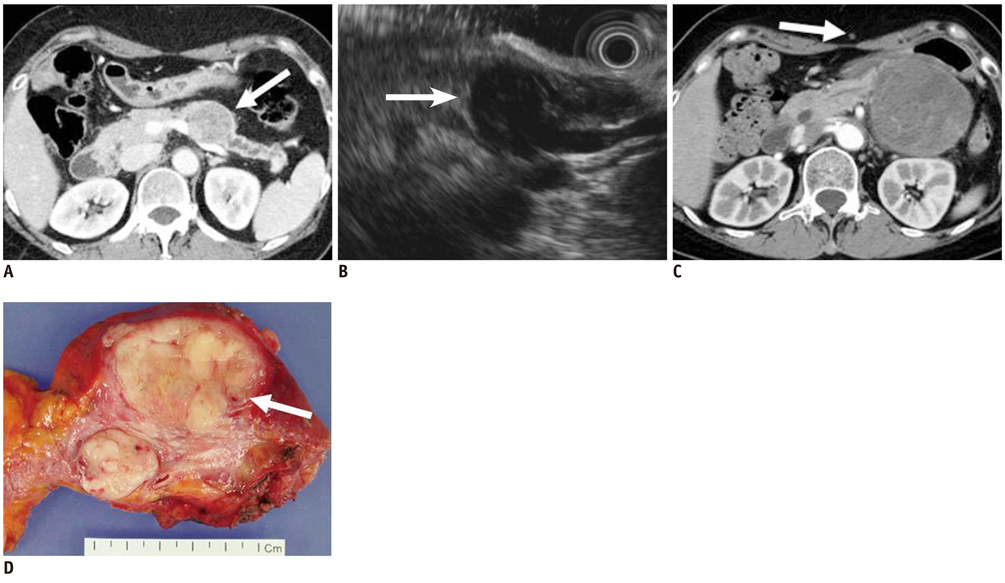
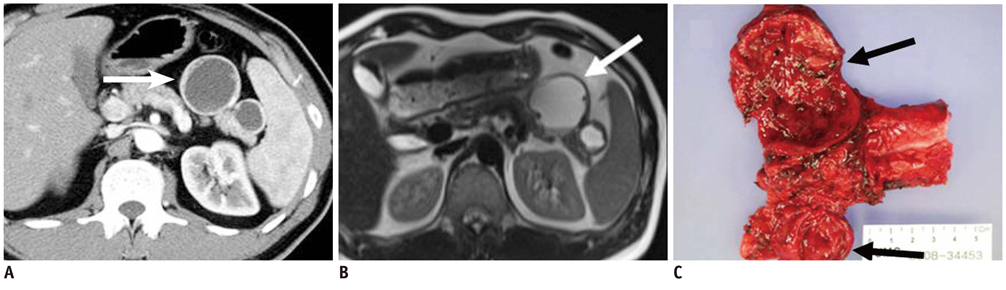
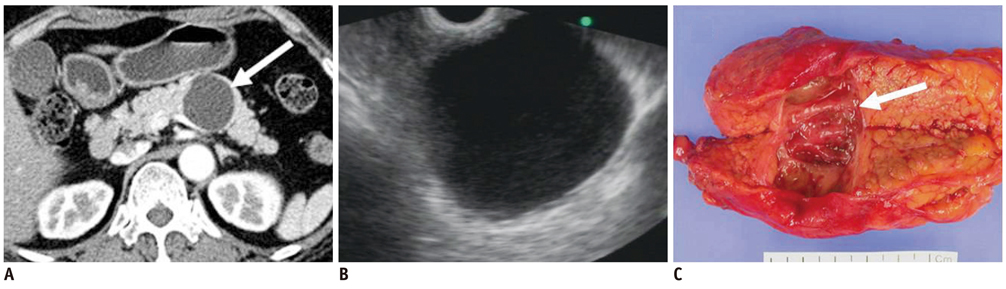
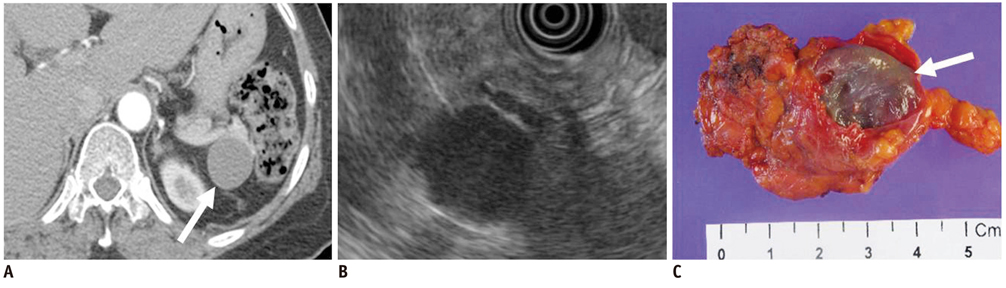
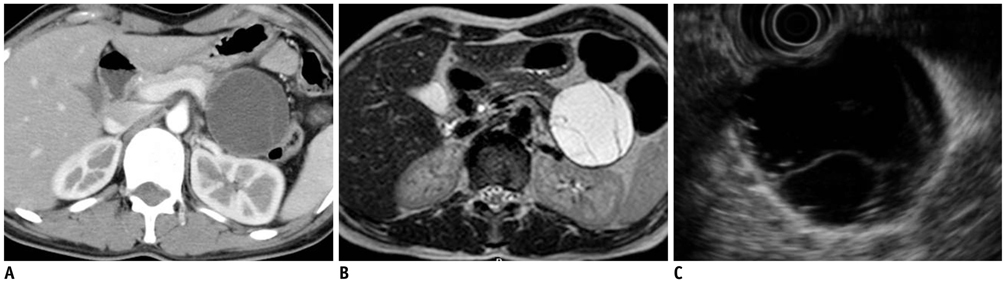
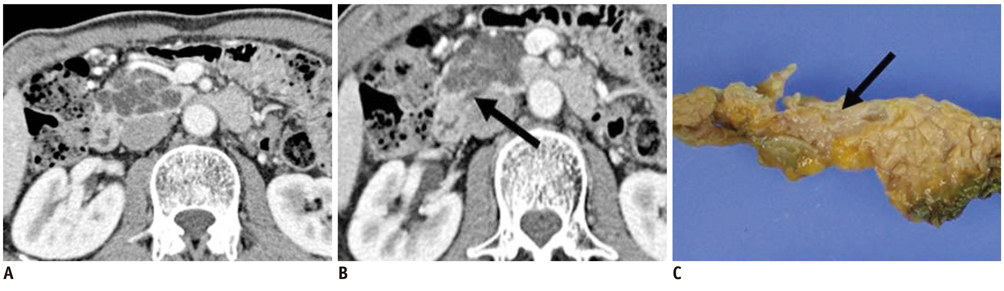
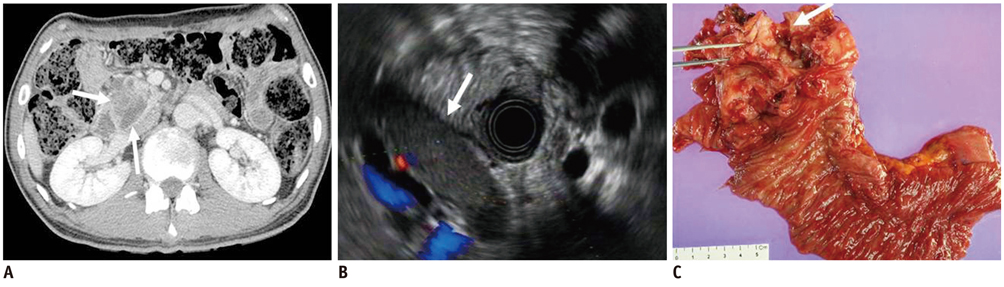
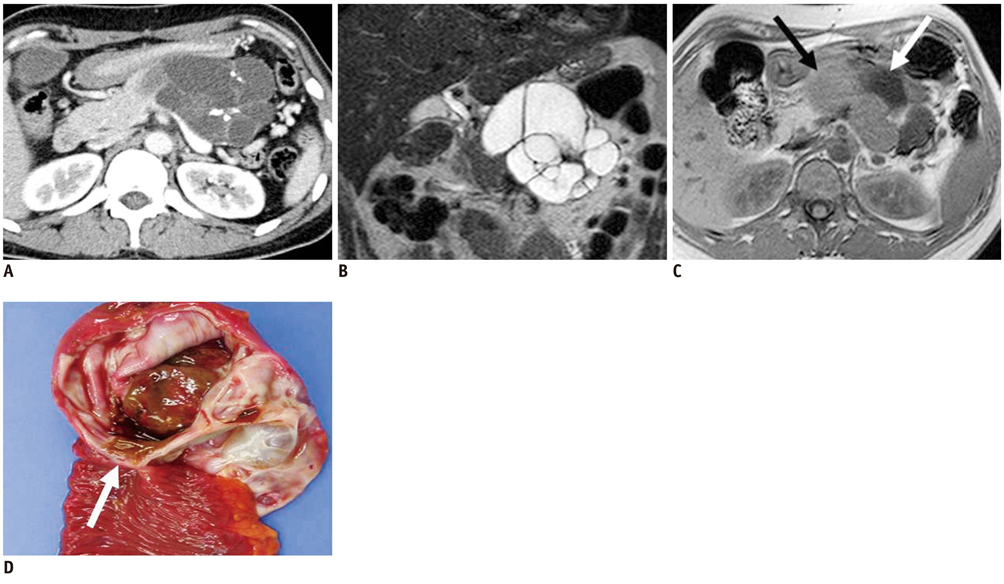
- Pancreatic tumors can be classified by their morphologic features on CT. The subtypes include solid tumors, mixed cystic and solid lesions, unilocular cysts, multilocular cystic lesions, and microcystic lesions. Endoscopic US and MRI can provide detailed information for classifying pancreatic lesions. Each subtype has different kinds of tumors and malignant potential, thus the classification can be useful for a better differential diagnosis and treatment planning. For this purpose, we suggest an appropriate modified classification system by using the imaging features of pancreatic tumors with an emphasis on CT findings and illustrate various findings of typical and atypical manifestations.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Retroperitoneal Extraskeletal Osteosarcoma without Calcification Mimicking Pancreas Tumor: CT Imaging of a Case Report
Jung Won Kim, Kyung Eun Bae, Kyeong Mee Park, Jae Hyung Kim, Myeong Ja Jeong, Soung Hee Kim, Ji Young Kim, Soo Hyun Kim, Mi Jin Kang, Ji Hae Lee, Tae Gyu Kim
J Korean Soc Radiol. 2018;78(5):340-344. doi: 10.3348/jksr.2018.78.5.340.
Reference
-
1. Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006. 187:1192–1198.2. Park MS, Kim KW, Lim JS, Lee JH, Kim JH, Kim SY, et al. Unusual cystic neoplasms in the pancreas: radiologic-pathologic correlation. J Comput Assist Tomogr. 2005. 29:610–616.3. Irie H, Honda H, Aibe H, Kuroiwa T, Yoshimitsu K, Shinozaki K, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000. 174:1403–1408.4. Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging. 2007. 26:86–93.5. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005. 25:1471–1484.6. Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997. 17:281–301.7. Lee LY, Hsu HL, Chen HM, Hsueh C. Ductal adenocarcinoma of the pancreas with huge cystic degeneration: a lesion to be distinguished from pseudocyst and mucinous cystadenocarcinoma. Int J Surg Pathol. 2003. 11:235–239.8. Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006. 187:W178–W186.9. Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003. 228:727–733.10. Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000. 175:99–103.11. Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005. 25:1451–1468. discussion 1468-1470.12. Levy MJ, Clain JE. Evaluation and management of cystic pancreatic tumors: emphasis on the role of EUS FNA. Clin Gastroenterol Hepatol. 2004. 2:639–653.13. Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc. 2009. 69:S203–S209.14. Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009. 193:136–142.15. Shintaku M, Arimoto A, Sakita N. Serous cystadenocarcinoma of the pancreas. Pathol Int. 2005. 55:436–439.16. Choi JY, Lee JM, Lee MW, Kim SJ, Choi SY, Kim JY, et al. Magnetic resonance pancreatography: comparison of two- and three-dimensional sequences for assessment of intraductal papillary mucinous neoplasm of the pancreas. Eur Radiol. 2009. 19:2163–2170.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Computed tomographic findings in posterior cranial fossa tumors: correlation between angiographic vascularityand CT enhancement
- CT in the diagnosis of pancreatic trauma
- Islet Cell Tumors of the Pancreas: A Variety of MultiphaseDynamic Imaging Findings with Pathologic Correlations Focusing on Nonfunctioning Tumors and Insulinomas
- Pathologic Features of Pancreatic Cystic Neoplasms
- Imaging Findings of Perineal Disease