Korean J Radiol.
2009 Oct;10(5):515-518. 10.3348/kjr.2009.10.5.515.
Inflammatory Pseudotumor of the Breast: a Case Report with Imaging Findings
- Affiliations
-
- 1Seoul National University Hospital Healthcare System Gangnam Center, Seoul 135-984, Korea.
- 2Department of Radiology and Clinical Research Institute, Seoul National University Hospital and the Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul 110-744, Korea. moonwk@radcom.snu.ac.kr
- 3Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea.
- KMID: 1093968
- DOI: http://doi.org/10.3348/kjr.2009.10.5.515
Abstract
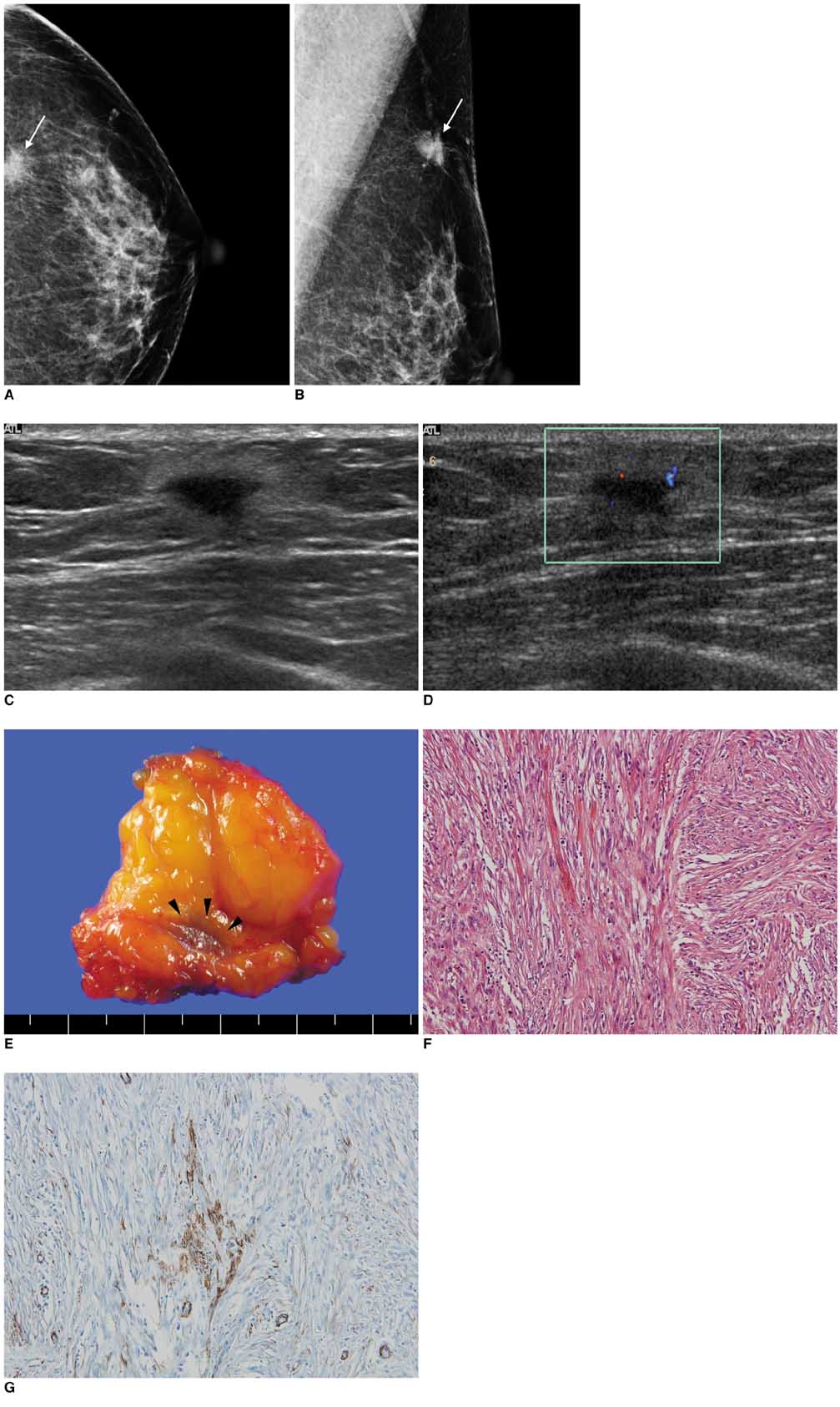
- Inflammatory pseudotumor, also known as inflammatory myofibroblastic tumor and plasma cell granuloma, is an uncommon low-grade lesion composed of spindle cells admixed with mature plasma cells and other inflammatory cells, such as histiocytes, lymphocytes, and eosinophils. Here, we describe the mammographic and ultrasonographic findings of a case of an inflammatory pseudotumor of the breast in a 60-year-old woman. With the suspicion of malignancy, core needle biopsy and surgical excision confirmed the mass as being an inflammatory pseudotumor of the breast.
Keyword
MeSH Terms
Figure
Reference
-
1. Haj M, Weiss M, Loberant N, Cohen I. Inflammatory pseudotumor of the breast: case report and literature review. Breast J. 2003. 9:423–425.2. Yip CH, Wong KT, Samuel D. Bilateral plasma cell granuloma (inflammatory pseudotumour) of the breast. Aust N Z J Surg. 1997. 67:300–302.3. Zardawi IM, Clark D, Williamsz G. Inflammatory myofibroblastic tumor of the breast. A case report. Acta Cytol. 2003. 47:1077–1081.4. Khanafshar E, Phillipson J, Schammel DP, Minobe L, Cymerman J, Weidner N. Inflammatory myofibroblastic tumor of the breast. Ann Diagn Pathol. 2005. 9:123–129.5. Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005. 113:66–69.6. Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, et al. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005. 29:275–278.7. Sastre-Garau X, Couturier J, Derre J, Aurias A, Klijanienko J, Lagace R. Inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast. Clinicopathological and genetic analysis of a case with evidence for clonality. J Pathol. 2002. 196:97–102.8. Chetty R, Govender D. Inflammatory pseudotumor of the breast. Pathology. 1997. 29:270–271.9. Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988. 90:627–632.10. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.11. Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990. 94:538–546.12. Maier HC, Sommers SC. Recurrent and metastatic pulmonary fibrous histiocytoma/plasma cell granuloma in a child. Cancer. 1987. 60:1073–1076.13. Cessna MH, Zhou H, Sanger WG, Perkins SL, Tripp S, Pickering D, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002. 15:931–938.14. Freeman A, Geddes N, Munson P, Joseph J, Ramani P, Sandison A, et al. Anaplastic lymphoma kinase (ALK1) staining and molecular analysis in inflammatory myofibroblastic tumours of the bladder: a preliminary clinicopathological study of nine cases and review of the literature. Mod Pathol. 2004. 17:765–771.15. Akbulut M, Gunhan-Bilgen I, Zekioglu O, Duygulu G, Oktay A, Ozdemir N. Fine needle aspiration cytology of inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast: a case report and review of the literature. Cytopathology. 2007. 18:384–387.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Inflammatory Pseudotumor Cerebri and Nasal Septum
- A Case of Inflammatory Pseudotumor in the Retroperitoneum
- Inflammatory Myofibroblastic Tumor of the Breast: A Case Report
- Inflammatory Pseudotumor in the Liver and Right Omentum Caused by Pelvic Inflammatory Disease: A Case Report
- Inflammatory Pseud0tumor of the Liver: A case report