J Vet Sci.
2006 Dec;7(4):327-332. 10.4142/jvs.2006.7.4.327.
Pharmacokinetics and bioavailability of doxycycline in ostriches (Struthio camelus) at two different dose rates
- Affiliations
-
- 1Department of Basic Veterinary Medical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan. abubasha@just.edu.jo
- 2Department of Pharmaceutical Technology, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan.
- KMID: 1089478
- DOI: http://doi.org/10.4142/jvs.2006.7.4.327
Abstract
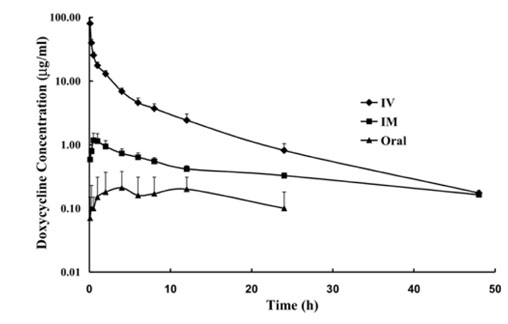
- A bioavailability and pharmacokinetics study of doxycycline was carried out on 30 healthy ostriches after a single intravenous (IV), intramuscular (IM) and oral dose of 15 mg/kg body weight. The plasma doxycycline concentration was determined by HPLC/UV at 0 (pretreatment), 0.08, 0.25, 0.5 1, 2, 4, 6, 8, 12, 24 and 48 h after administration. The plasma concentration-time curves were examined using non-compartmental methods based on the statistical moment theory for only the higher dose. After IV administration, the elimination half-life (t(1/2beta)), mean residence time (MRT), volume of distribution at the steady-state (V(ss)), volume of distribution (Vd(area)) and total body clearance (Cl(B)) were 7.67 +/- 0.62 h, 6.68 +/- 0.86 h, 0.86 +/- 0.16 l/kg, 1.67 +/- 0.52 l/kg and 2.51 +/- 0.63 ml/min/kg, respectively. After IM and oral dosing, the mean peak plasma concentrations (C(max)) were 1.34 +/- 0.33 and 0.30 +/- 0.04 microgram/ml, respectively, which were achieved at a postadministration time (t(max)) of 0.75 +/- 0.18, 3.03 +/- 0.48 h, respectively. The t(1/2beta), Vd(area) and Cl(B) after IM administration were 25.02 +/- 3.98 h, 23.99 +/- 3.4 l/kg and 12.14 +/- 1.71 ml/min/kg, respectively and 19.25 +/- 2.53 h, 61.49 +/- 7 l/kg and 40.19 +/- 3.79 ml/min/kg after oral administration, respectively. The absolute bioavailability (F) of doxycycline was 5.03 and 17.52% after oral and IM administration, respectively. These results show that the dose data from other animals particularly mammals cannot be extrapolated to ostriches. Therefore, based on these results along with those reported in the literature, further studies on the pharmacokinetic/pharmacodynamic, in vitro minimum inhibitory concentration values and clinical applications of doxycycline in ostriches are required.
Keyword
MeSH Terms
-
Administration, Oral
Animals
Anti-Bacterial Agents/administration & dosage/blood/*pharmacokinetics
Area Under Curve
Biological Availability
Dose-Response Relationship, Drug
Doxycycline/administration & dosage/blood/*pharmacokinetics
Half-Life
Injections, Intramuscular/veterinary
Injections, Intravenous/veterinary
Struthioniformes/*metabolism
Figure
Reference
-
1. Axisa B, Naylor AR, Bell PR, Thompson MM. Simple and reliable method of doxycycline determination in human plasma and biological tissues. J Chromatogr B Biomed Appl. 2000. 744:359–365.
Article2. Abd El-Aty AM, Goudah A, Zhou HH. Pharmacokinetics of doxycycline after administration as a single intravenous bolus and intramuscular doses to non-lactating Egyptian goats. Pharmacol Res. 2004. 49:487–491.
Article3. Anadón A, Martínez-Larrañaga MR, Díaz MJ, Bringas P, Fernández MC, Fernández-Cruz ML, Iturbe J, Martinez MA. Pharmacokinetics of doxycycline in broiler chickens. Avian Pathol. 1994. 23:79–90.
Article4. Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980. 176:1061–1068.5. Baert K, Croubels S, Gasthuys F, De Busser J, Backer P. Pharmacokinetics and oral bioavailability of a doxycycline formulation (doxycycline 75%) in non-fasted young pigs. J Vet Pharmacol Ther. 2000. 23:45–48.
Article6. Bezuidenhout AJ. The topography of the thoraco-abdominal viscera in the ostrich (Struthio camelus). Onderstepoort J Vet Res. 1986. 53:111–117.7. Bousquet E, Morvan H, Aitken I, Morgan JH. Comparative in vitro activity of doxycycline and oxytetracycline against porcine respiratory pathogens. Vet Rec. 1997. 141:37–40.
Article8. Bryant JE, Brown MP, Gronwall RR, Merritt KA. Study of intragastric administration of doxycycline: pharmacokinetics including body fluid, endometrial and minimum inhibitory concentrations. Equine Vet J. 2000. 32:233–238.
Article9. Butaye P, Ducatelle R, De Backer P, Vermeersch H, Remon JP, Haesebrouck F. In vitro activities of doxycycline and enrofloxacin against European Chlamydia psittaci strains from turkeys. Antimicrob Agents Chemother. 1997. 41:2800–2801.
Article10. Cho P, Brown R, Anderson M. Comparative gross anatomy of ratites. Zoo Biol. 1984. 3:133–144.
Article11. Croubels S, Baert K, De Busser J, De Backer P. Residue study of doxycycline and 4-epidoxycycline in pigs medicated via drinking water. Analyst. 1998. 123:2733–2736.
Article12. Davis JL, Salmon JH, Papich MG. Pharmacokinetics and tissue distribution of doxycycline after oral administration of single and multiple doses in horses. Am J Vet Res. 2006. 67:310–316.
Article13. Gibaldi M, Perrier D. Pharmacokinetics. 1982. 2nd ed. New York: Marcel Dekker;409–417.14. Hoelscher AA, Bahcall JK, Maki JS. In vitro evaluation of the antimicrobial effects of a root canal sealer-antibiotic combination against Enterococcus faecalis. J Endod. 2006. 32:145–147.
Article15. Ismail MM, El-Kattan YA. Disposition kinetics of doxycycline in chickens naturally infected with Mycoplasma gallisepticum. Br Poult Sci. 2004. 45:550–556.
Article16. Jensen JM. Current ratite therapy. Vet Clin North Am Food Anim Pract. 1998. 14:484–502.
Article17. Jha VK, Jayachandran C, Singh MK, Singh SD. Pharmacokinetic data on doxycycline and its distribution in different biological fluids in female goats. Vet Res Commun. 1989. 13:11–16.
Article18. Laczay P, Semjen G, Lehel J, Nagy G. Pharmacokinetics and bioavailability of doxycycline in fasted and nonfasted broiler chickens. Acta Vet Hung. 2001. 49:31–37.
Article19. McKellar QA, Sanchez Bruni SF, Jones DG. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther. 2004. 27:503–514.
Article20. Meijer LA, Ceyssens KG, de Greve BI, de Bruijn W. Pharmacokinetics and bioavailability of doxycycline hyclate after oral administration in calves. Vet Q. 1993. 15:1–5.
Article21. Mittermayer H, Rotter M, Riezinger F, Thiel W, Stanek G. Susceptibility of the Bacteroides fragilis group to 10 antibiotics. Results of 4 laboratories in Austria. Zentralbl Bakteriol Mikrobiol Hyg (A). 1986. 262:500–511.22. Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004. 42:1915–1922.
Article23. Ole-Mapenay IM, Mitema ES, Maitho TE. Aspects of pharmacokinetics of doxycycline given to healthy and pneumonic East African dwarf goats by intramuscular injection. Vet Res Commun. 1997. 21:453–462.24. Pijpers A, Van Klingeren B, Schoevers EJ, Verheijden JHM, Van Miert AS. In vitro activity of five tetracyclines and some other antimicrobial agents against four porcine respiratory tract pathogens. J Vet Pharmacol Ther. 1989. 12:267–276.
Article25. Prats C, El Korchi G, Giralt M, Cristofol C, Pena J, Zorrilla I, Saborit J, Perez B. PK and PK/PD of doxycycline in drinking water after therapeutic use in pigs. J Vet Pharmacol Ther. 2005. 28:525–530.
Article26. Riond JL, Riviere JE. Pharmacokinetics and metabolic inertness of doxycycline in young pigs. Am J Vet Res. 1990. 51:1271–1275.27. Riond JL, Tyczkowska K, Riviere JE. Pharmacokinetics and metabolic inertness of doxycycline in calves with mature or immature rumen function. Am J Vet Res. 1989. 50:1329–1333.28. Riond JL, Vaden S, Riviere JE. Comparative pharmacokinetics of doxycycline in cats and dogs. J Vet Pharmacol Ther. 1990. 13:415–424.
Article29. Santos MD, Vermeersch H, Remon JP, Schelkens M, De Backer P, Van Bree HJ, Ducatelle R, Haesebrouck F. Pharmacokinetics and bioavailability of doxycycline in turkeys. J Vet Pharmacol Ther. 1996. 19:274–280.
Article30. Shane SM. Infectious diseases and parasites of ratites. Vet Clin North Am Food Anim Pract. 1998. 14:455–483.
Article31. Tanswell P, Koup J. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993. 31:514–520.32. Verwoerd DJ. Ostrich diseases. Rev Sci Tech. 2000. 19:638–661.
Article33. Waites KB, Crabb DM, Duffy LB. Comparative in vitro susceptibilities and bactericidal activities of investigational fluoroquinolone ABT-492 and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2003. 47:3973–3975.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The stable isotope method for determining absolute bioavailability
- Comparative Study of First-in-Human Dose Estimation Approaches using Pharmacometrics
- Doxycycline-induced Staining of Adult Teeth
- Doxytycline in Treatment of Acute Gonococcal Uriethritis
- Bioavailability Assessment of Isoflavones between Soybean and Soybean Sprout in Rat