Korean J Radiol.
2011 Apr;12(2):232-240. 10.3348/kjr.2011.12.2.232.
Concordant or Discordant? Imaging-Pathology Correlation in a Sonography-Guided Core Needle Biopsy of a Breast Lesion
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 120-752, Korea. ekkim@yuhs.ac
- 2Department of Radiology, Bundang CHA Hospital, CHA University, Gyeonggi-do 463-712, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 4Department of Pathology, Bundang CHA Hospital, CHA University, Gyeonggi-do 463-712, Korea.
- KMID: 1088569
- DOI: http://doi.org/10.3348/kjr.2011.12.2.232
Abstract
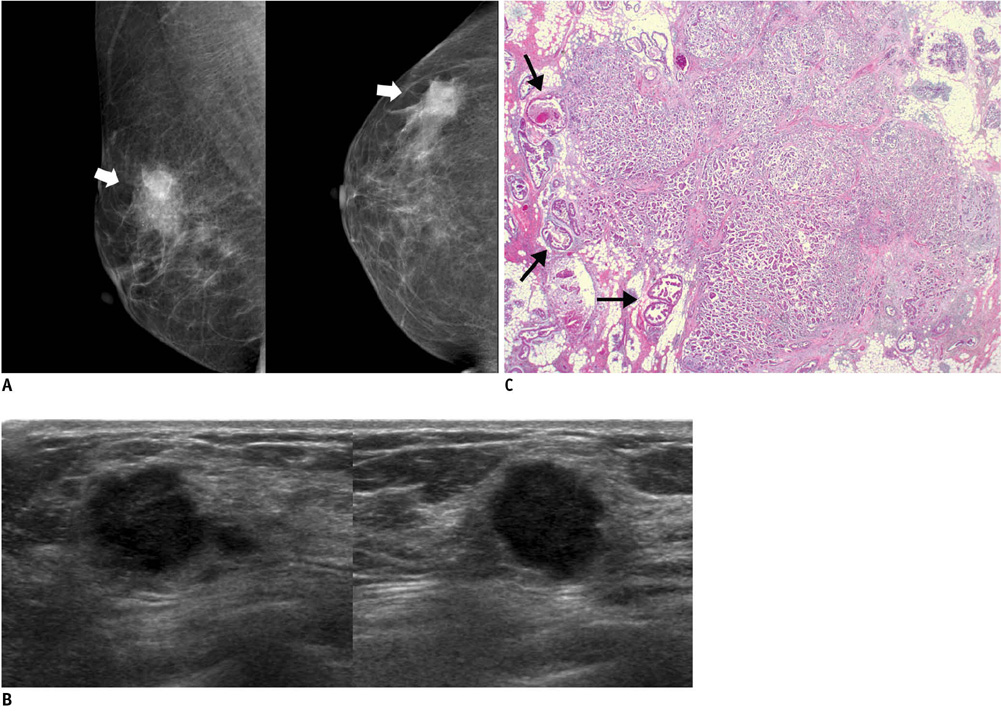
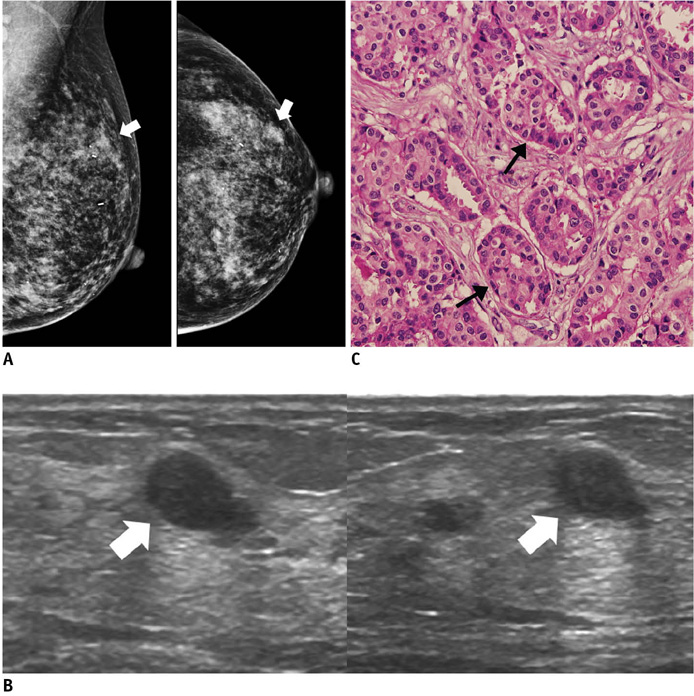
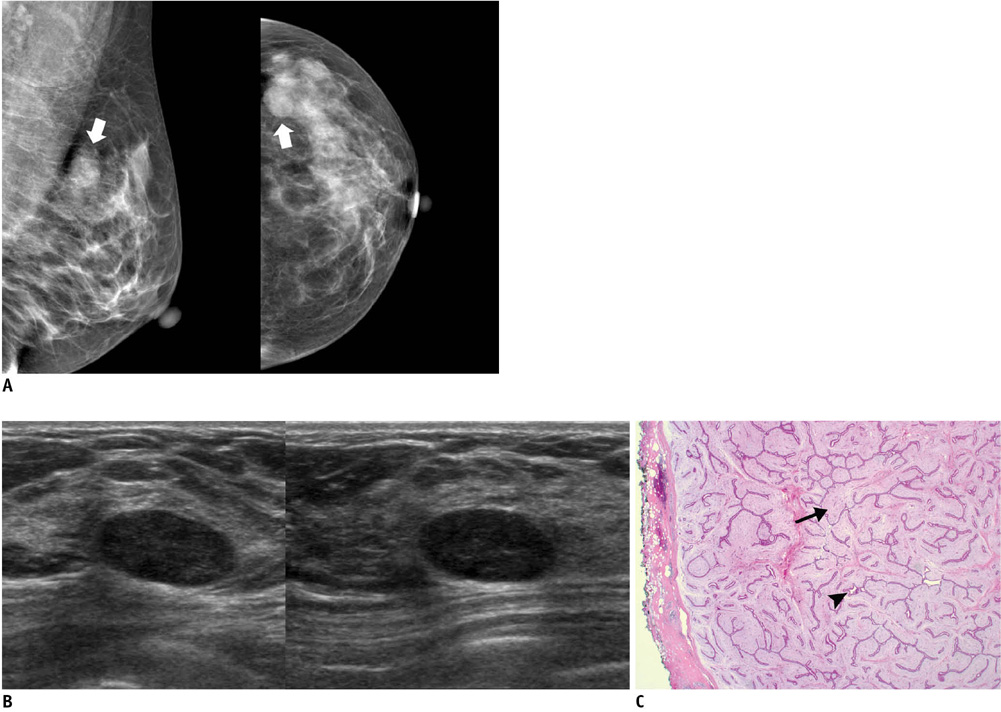
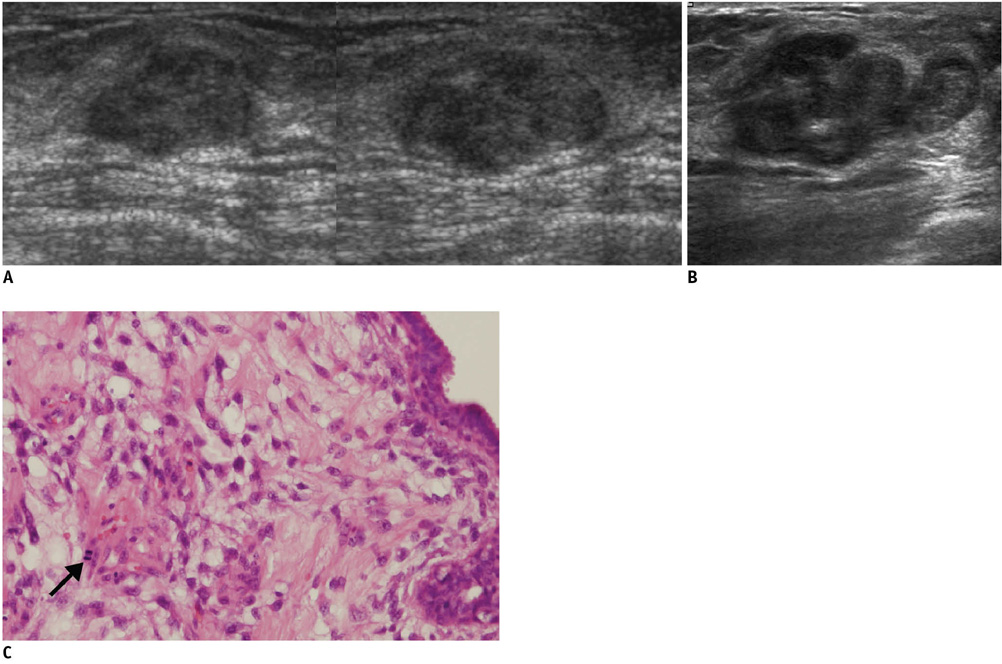
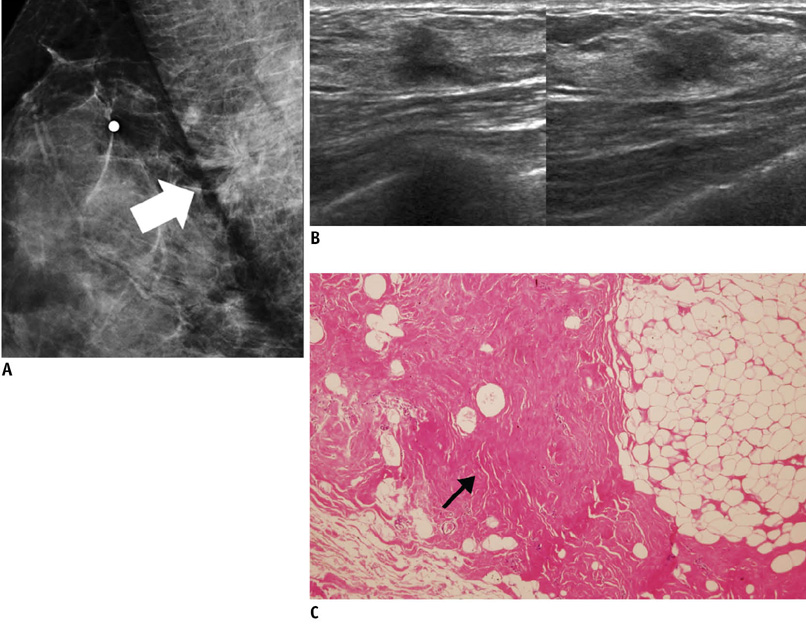
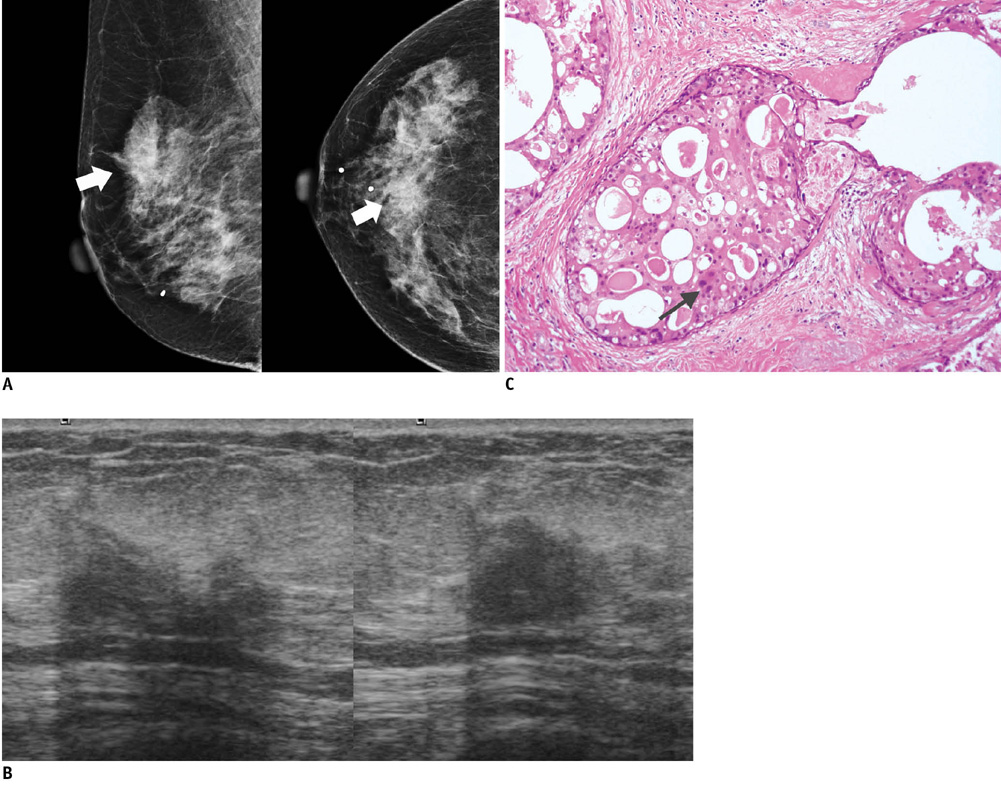
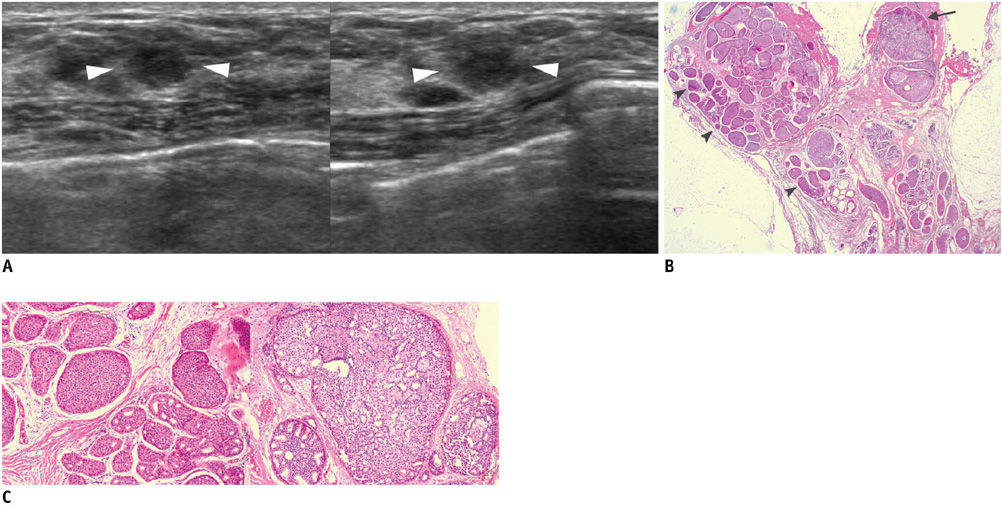
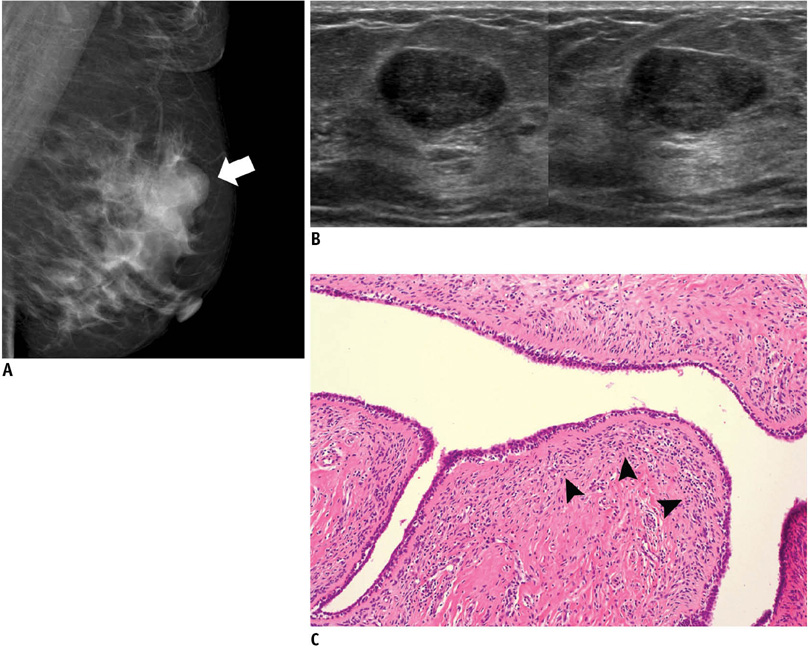
- An imaging-guided core needle biopsy has been proven to be reliable and accurate for the diagnosis of both benign and malignant diseases of the breast, and has replaced surgical biopsy. However, the possibility of a false-negative biopsy still remains. Imaging-pathology correlation is of critical importance in imaging-guided breast biopsies to detect such a possible sampling error and avoid a delay in diagnosis. We will review five possible categories and corresponding management after performing an imaging-pathology correlation in a sonography-guided core needle biopsy of a breast lesion, as well as illustrate the selected images for each category in conjunction with the pathologic finding. Radiologists should be familiar with the imaging features of various breast pathologies and be able to appropriately correlate imaging findings with pathologic results after a core needle biopsy.
Keyword
MeSH Terms
Figure
Reference
-
1. Bassett LW, Mahoney MC, Apple SK. Interventional breast imaging: current procedures and assessing for concordance with pathology. Radiol Clin North Am. 2007. 45:881–894.2. Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics. 2007. 27:79–94.3. Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000. 89:2538–2546.4. Parikh J, Tickman R. Image-guided tissue sampling: where radiology meets pathology. Breast J. 2005. 11:403–409.5. Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002. 40:483–500.6. Comstock CE. Feig SA, editor. US-guided interventional procedures. 2005 Syllabus: categorical course in diagnostic radiology-breast imaging. 2005. Oak Brook, IL: Radiological Society of North America;155–168.7. Whitman GJ, Erguvan-Dogan B, Yang WT, Wilson J, Patel P, Krishnamurthy S. Ultrasound-guided breast biopsies. Ultrasound Clin. 2006. 1:603–615.8. Starvros AT. McAllister L, Donnellan K, Martin SP, Rothschild R, editors. False-negative and false-positive examinations. Breast ultrasound. 2004. Philadelphia, PA: Lippincott Williams & Wilkins;947–978.9. Kim MJ, Kim EK, Lee JY, Youk JH, Park BW, Kim SI, et al. Breast lesions with imaging-histologic discordance during US-guided 14G automated core biopsy: can the directional vacuum-assisted removal replace the surgical excision? Initial findings. Eur Radiol. 2007. 17:2376–2383.10. Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol. 2008. 190:1209–1215.11. Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006. 239:385–391.12. Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management--follow-up and outcome. Radiology. 2008. 248:773–781.13. Lee HJ, Kim EK, Kim MJ, Youk JH, Lee JY, Kang DR, et al. Observer variability of Breast Imaging Reporting and Data System (BI-RADS) for breast ultrasound. Eur J Radiol. 2008. 65:293–298.14. American College of Radiology. American College of Radiology. Breast imaging reporting and data system-mammography. Breast imaging reporting and data system. 2003. 4th ed. Reston, VA: American College of Radiology.15. Bent CK, Bassett LW, D'Orsi CJ, Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR Am J Roentgenol. 2010. 194:1378–1383.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Breast Lesions with Discordant Results on Ultrasound-guided Core Needle Biopsy
- Sonographically-Guided 14-Gauge Core Needle Biopsy for Papillary Lesions of the Breast
- Clinicopathologic Features of the Papillary Breast Lesions Diagnosed on Ultrasonography-guided Core Needle Biopsy
- The Clinical Experience of an Ultrasound-guided Vacuum-assisted Resection (Mammotome) for Benign Breast Lesions through a Core Needle Biopsy
- Pseudoaneurysm of the Breast after Core Needle Biopsy: A Case Report