J Vet Sci.
2011 Sep;12(3):221-226. 10.4142/jvs.2011.12.3.221.
Determination of staphylococcal exotoxins, SCCmec types, and genetic relatedness of Staphylococcus intermedius group isolates from veterinary staff, companion animals, and hospital environments in Korea
- Affiliations
-
- 1Department of Microbiology, College of Veterinary Medicine and BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea. koohj@snu.ac.kr
- 2National Veterinary Research and Quarantine Service, Ministry of Agriculture and Forestry, Anyang 430-856, Korea.
- KMID: 1067393
- DOI: http://doi.org/10.4142/jvs.2011.12.3.221
Abstract
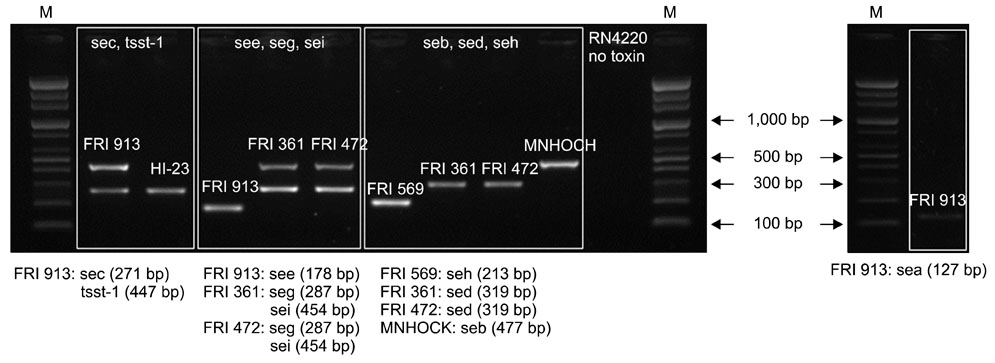
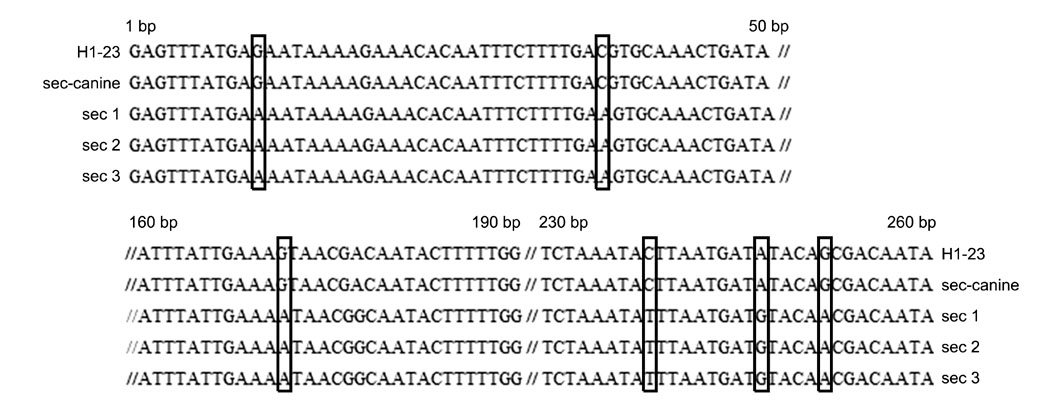
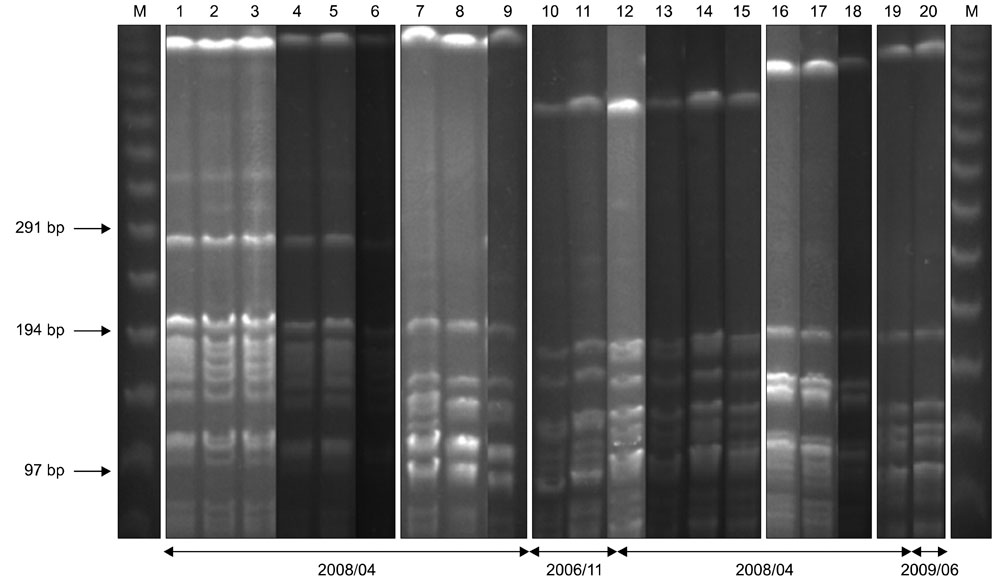
- The Staphylococcus (S.) intermedius group (SIG) has been a main research subject in recent years. S. pseudintermedius causes pyoderma and otitis in companion animals as well as foodborne diseases. To prevent SIG-associated infection and disease outbreaks, identification of both staphylococcal exotoxins and staphylococcal cassette chromosome mec (SCCmec) types among SIG isolates may be helpful. In this study, it was found that a single isolate (one out of 178 SIG isolates examined) harbored the canine enterotoxin SEC gene. However, the S. intermedius exfoliative toxin gene was found in 166 SIG isolates although the S. aureus-derived exfoliative toxin genes, such as eta, etb and etd, were not detected. SCCmec typing resulted in classifying one isolate as SCCmec type IV, 41 isolates as type V (including three S. intermedius isolates), and 10 isolates as non-classifiable. Genetic relatedness of all S. pseudintermedius isolates recovered from veterinary staff, companion animals, and hospital environments was determined by pulsed-field gel electrophoresis. Strains having the same band patterns were detected in S. pseudintermedius isolates collected at 13 and 18 months, suggesting possible colonization and/or expansion of a specific S. pseudintermedius strain in a veterinary hospital.
Keyword
MeSH Terms
-
Animals
Bacterial Toxins/genetics/metabolism
Cat Diseases/epidemiology/*microbiology
Cats
Chromosomes, Bacterial/genetics/metabolism
Dog Diseases/epidemiology/*microbiology
Dogs
Electrophoresis, Gel, Pulsed-Field/veterinary
Enterotoxins/genetics/metabolism
Exfoliatins/genetics/metabolism
Exotoxins/*genetics/metabolism
Hospitals, Animal
Humans
Medical Staff, Hospital
Molecular Sequence Data
Pets/microbiology
Polymerase Chain Reaction/veterinary
Republic of Korea/epidemiology
Staphylococcal Infections/epidemiology/microbiology/*veterinary
Staphylococcus/genetics/isolation & purification
Staphylococcus intermedius/*genetics/*isolation & purification
Figure
Reference
-
1. Balaban N, Rasooly A. Staphylococcal enterotoxins. Int J Food Microbiol. 2000. 61:1–10.
Article2. Bannoehr J, Franco A, Iurescia M, Battisti A, Fitzgerald JR. Molecular diagnostic identification of Staphylococcus pseudintermedius. J Clin Microbiol. 2009. 47:469–471.
Article3. Baron F, Cochet MF, Pellerin JL, Ben Zakour N, Lebon A, Navarro A, Proudy I, Le Loir Y, Gautier M. Development of a PCR test to differentiate between Staphylococcus aureus and Staphylococcus intermedius. J Food Prot. 2004. 67:2302–2305.
Article4. Boye K, Bartels MD, Andersen IS, Møller JA, Westh H. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin Microbiol Infect. 2007. 13:725–727.
Article5. Chuang CY, Yang YL, Hsueh PR, Lee PI. Catheter-related bacteremia caused by Staphylococcus pseudintermedius refractory to antibiotic-lock therapy in a hemophilic child with dog exposure. J Clin Microbiol. 2010. 48:1497–1498.
Article6. Frank LA, Kania SA, Kirzeder EM, Eberlein LC, Bemis DA. Risk of colonization or gene transfer to owners of dogs with meticillin-resistant Staphylococcus pseudintermedius. Vet Dermatol. 2009. 20:496–501.
Article7. Futagawa-Saito K, Makino S, Sunaga F, Kato Y, Sakurai-Komada N, Ba-Thein W, Fukuyasu T. Identification of first exfoliative toxin in Staphylococcus pseudintermedius. FEMS Microbiol Lett. 2009. 301:176–180.
Article8. Herschleb J, Ananiev G, Schwartz DC. Pulsed-field gel electrophoresis. Nat Protoc. 2007. 2:677–684.
Article9. Higuchi W, Takano T, Teng LJ, Yamamoto T. Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun. 2008. 377:752–756.
Article10. Ishihara K, Shimokubo N, Sakagami A, Ueno H, Muramatsu Y, Kadosawa T, Yanagisawa C, Hanaki H, Nakajima C, Suzuki Y, Tamura Y. Occurrence and molecular characteristics of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in an academic veterinary hospital. Appl Environ Microbiol. 2010. 76:5165–5174.
Article11. Kadlec K, Schwarz S, Perreten V, Andersson UG, Finn M, Greko C, Moodley A, Kania SA, Frank LA, Bemis DA, Franco A, Iurescia M, Battisti A, Duim B, Wagenaar JA, van Duijkeren E, Weese JS, Fitzgerald JR, Rossano A, Guardabassi L. Molecular analysis of methicillin-resistant Staphylococcus pseudintermedius of feline origin from different European countries and North America. J Antimicrob Chemother. 2010. 65:1826–1828.
Article12. Lautz S, Kanbar T, Alber J, Lämmler C, Weiss R, Prenger-Berninghoff E, Zschöck M. Dissemination of the gene encoding exfoliative toxin of Staphylococcus intermedius among strains isolated from dogs during routine microbiological diagnostics. J Vet Med B Infect Dis Vet Public Health. 2006. 53:434–438.
Article13. Lim SK, Nam HM, Park HJ, Lee HS, Choi MJ, Jung SC, Lee JY, Kim YC, Song SW, Wee SH. Prevalence and characterization of methicillin-resistant Staphylococcus aureus in raw meat in Korea. J Microbiol Biotechnol. 2010. 20:775–778.14. Loeffler A, Linek M, Moodley A, Guardabassi L, Sung JML, Winkler M, Weiss R, Lloyd DH. First report of multiresistant, mecA-positive Staphylococcus intermedius in Europe: 12 cases from a veterinary dermatology referral clinic in Germany. Vet Dermatol. 2007. 18:412–421.
Article15. McClure JA, Conly JM, Elsayed S, Zhang K. Multiplex PCR assay to facilitate identification of the recently described staphylococcal cassette chromosome mec type VIII. Mol Cell Probes. 2010. 24:229–232.
Article16. Nakaminami H, Noguchi N, Ikeda M, Hasui M, Sato M, Yamamoto S, Yoshida T, Asano T, Senoue M, Sasatsu M. Molecular epidemiology and antimicrobial susceptibilities of 273 exfoliative toxin-encoding-gene-positive Staphylococcus aureus isolates from patients with impetigo in Japan. J Med Microbiol. 2008. 57:1251–1258.
Article17. Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O'Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002. 40:4289–4294.
Article18. Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002. 46:2155–2161.
Article19. Omoe K, Ishikawa M, Shimoda Y, Hu DL, Ueda S, Shinagawa K. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J Clin Microbiol. 2002. 40:857–862.20. Omoe K, Hu DL, Takahashi-Omoe H, Nakane A, Shinagawa K. Comprehensive analysis of classical and newly described staphylococcal superantigenic toxin genes in Staphylococcus aureus isolates. FEMS Microbiol Lett. 2005. 246:191–198.
Article21. Perreten V, Kadlec K, Schwarz S, Grönlund Andersson U, Finn M, Greko C, Moodley A, Kania SA, Frank LA, Bemis DA, Franco A, Iurescia M, Battisti A, Duim B, Wagenaar JA, van Duijkeren E, Weese JS, Fitzgerald JR, Rossano A, Guardabassi L. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: an international multicentre study. J Antimicrob Chemother. 2010. 65:1145–1154.
Article22. Ruscher C, Lübke-Becker A, Semmler T, Wleklinski CG, Paasch A, Šoba A, Stamm I, Kopp P, Wieler LH, Walther B. Widespread rapid emergence of a distinct methicillin- and multidrug-resistant Staphylococcus pseudintermedius (MRSP) genetic lineage in Europe. Vet Microbiol. 2010. 144:340–346.
Article23. Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J Clin Microbiol. 2007. 45:1118–1125.
Article24. Sato H, Matsumori Y, Tanabe T, Saito H, Shimizu A, Kawano J. A new type of staphylococcal exfoliative toxin from a Staphylococcus aureus strain isolated from a horse with phlegmon. Infect Immun. 1994. 62:3780–3785.
Article25. Sila J, Sauer P, Kolar M. Comparison of the prevalence of genes coding for enterotoxins, exfoliatins, panton-valentine leukocidin and tsst-1 between methicillin-resistant and methicillin-susceptible isolates of Staphylococcus aureus at the university hospital in Olomouc. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009. 153:215–218.
Article26. Stegmann R, Burnens A, Maranta CA, Perreten V. Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J Antimicrob Chemother. 2010. 65:2047–2048.
Article27. Terauchi R, Sato H, Hasegawa T, Yamaguchi T, Aizawa C, Maehara N. Isolation of exfoliative toxin from Staphylococcus intermedius and its local toxicity in dogs. Vet Microbiol. 2003. 94:19–29.
Article28. Van Hoovels L, Vankeerberghen A, Boel A, Van Vaerenbergh K, De Beenhouwer H. First case of Staphylococcus pseudintermedius infection in a human. J Clin Microbiol. 2006. 44:4609–4612.
Article29. Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. 2010. 140:418–429.
Article30. Yoo JH, Yoon JW, Lee SY, Park HM. High prevalence of Fluoroquinolone- and Methicillin-resistant Staphylococcus pseudintermedius isolates from canine pyoderma and otitis externa in veterinary teaching hospital. J Microbiol Biotechnol. 2010. 20:798–802.31. Yoon JW, Lee GJ, Lee SY, Park C, Yoo JH, Park HM. Prevalence of genes for enterotoxins, toxic shock syndrome toxin 1 and exfoliative toxin among clinical isolates of Staphylococcus pseudintermedius from canine origin. Vet Dermatol. 2010. 21:484–489.
Article32. Youn JH, Hwang SY, Kim SH, Koo HC, Shin S, Lim SK, Park YH. mecA gene transferrability and antibiogram of zoonotic Staphylococcus intermedius from animals, staff and the environment in animal hospitals in Korea. J Microbiol Biotechnol. 2010. 20:425–432.33. Youn JH, Yoon JW, Koo HC, Lim SK, Park YH. Prevalence and antimicrogram of Staphylococcus intermedius group isolates from veterinary staff, companion animals, and the environment in veterinary hospitals in Korea. J Vet Diagn Invest. 2011. 23:268–274.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Epidemiology of Methicillin-Resistant Staphylococcus aureus Isolates with Toxic Shock Syndrome Toxin and Staphylococcal Enterotoxin C genes
- Correlation Between Staphylococcal Cassette Chromosome mec Type and Coagulase Serotype of Methicillin-Resistant Staphylococcus aureus
- Genetic characterization of methicillin-resistant Staphylococcus aureus from humans and animals within the community
- Dissimilarity of ccrAB gene sequences between methicillin-resistant Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus among bovine isolates in Korea
- Clonal Types and Antimicrobial Susceptibilitiy Patterns in Methicillin-Resistant Staphylococcus aureus Isolates from a Korean Hospital