Korean J Ophthalmol.
2011 Aug;25(4):231-237. 10.3341/kjo.2011.25.4.231.
Combined Treatment of Photodynamic Therapy and Bevacizumab for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration
- Affiliations
-
- 1Department of Ophthalmology, Inje University College of Medicine, Busan, Korea.
- 2Department of Ophthalmology, Pusan National University School of Medicine, Yangsan, Korea. jlee@pusan.ac.kr
- 3Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 1018409
- DOI: http://doi.org/10.3341/kjo.2011.25.4.231
Abstract
- PURPOSE
To evaluate the outcome of a combined photodynamic therapy and intravitreal injection of bevacizumab in choroidal neovascularization secondary to age-related macular degeneration.
METHODS
Photodynamic therapy (PDT) was administered to 28 eyes followed by 3 consecutive bevacizumab injections. Patients were followed-up for more than 12 months. At baseline, 1, 3, 6, and 12 months post PDT, visual acuity (VA) and central macular thickness were measured using optical coherence tomography.
RESULTS
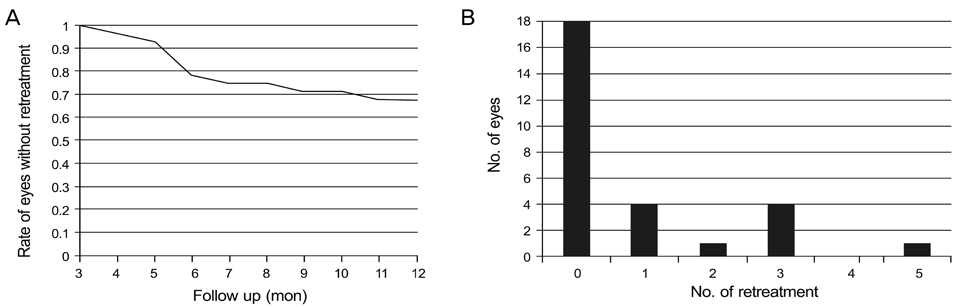
The mean VA was significantly improved from logarithm of the minimal angle of resolution 0.86 at baseline to 0.69 at 1 month (p = 0.011), 0.63 at 3 months (p = 0.003), 0.64 at 6 months (p = 0.004) and 0.60 at 12 months (p < 0.001). Central macular thickness decreased significantly from 328.3 microm at baseline to 230.0 microm at 6 months and 229.9 microm at 1 year (p < 0.001). Reinjection mean number was 0.4 for 6 months and 0.8 for 12 months. By 1 year, retreatment was performed in 10 eyes (36%).
CONCLUSIONS
PDT combined with three consecutive intraviteal bevacizumab injections was effective in improving VA and reducing central macular thickness.
Keyword
MeSH Terms
-
Aged
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal, Humanized/*administration & dosage
Choroidal Neovascularization/diagnosis/*drug therapy/etiology
Dose-Response Relationship, Drug
Drug Therapy, Combination
Female
Fluorescein Angiography
Follow-Up Studies
Fundus Oculi
Humans
Intravitreal Injections
Macula Lutea/drug effects/*pathology
Macular Degeneration/*complications/diagnosis/drug therapy
Male
Photochemotherapy/*methods
Photosensitizing Agents/administration & dosage
Porphyrins/*administration & dosage
Retrospective Studies
Tomography, Optical Coherence
Treatment Outcome
Visual Acuity
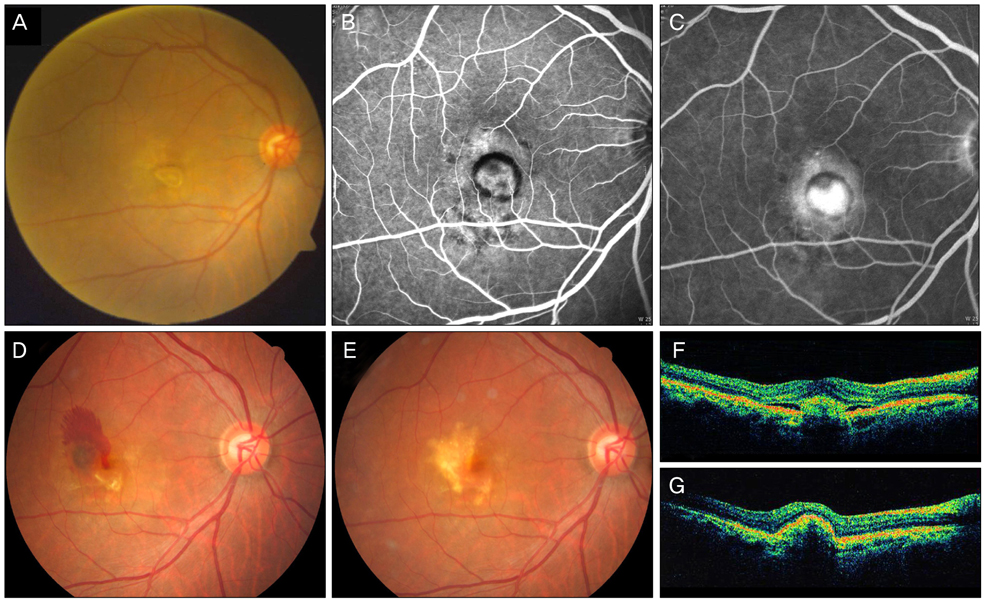
Figure
Reference
-
1. Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001. 131:541–560.2. Azab M, Boyer DS, Bressler NM, et al. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol. 2005. 123:448–457.3. Bressler NM. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001. 119:198–207.4. Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci. 2002. 43:830–841.5. Matsuoka M, Ogata N, Otsuji T, et al. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004. 88:809–815.6. Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003. 44:4473–4480.7. Schmidt-Erfurth U, Miller JW, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of retreatments in a phase 1 and 2 study. Arch Ophthalmol. 1999. 117:1177–1187.8. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363–372.e5.9. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006. 26:383–390.10. Bashshur ZF, Bazarbachi A, Schakal A, et al. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006. 142:1–9.11. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006. 47:4569–4578.12. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006. 26:495–511.13. Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004. 351:2805–2816.14. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006. 355:1419–1431.15. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006. 355:1432–1444.16. Navea A, Mataix J, Desco MC, et al. One-year follow-up of combined customized therapy. Photodynamic therapy and bevacizumab for exudative age-related macular degeneration. Retina. 2009. 29:13–19.17. Kaiser PK. Verteporfin photodynamic therapy and anti-angiogenic drugs: potential for combination therapy in exudative age-related macular degeneration. Curr Med Res Opin. 2007. 23:477–487.18. Kaiser PK, Blodi BA, Shapiro H, et al. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007. 114:1868–1875.19. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007. 144:850–857.20. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials: TAP report. Arch Ophthalmol. 1999. 117:1329–1345.21. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009. 148:43–58.e1.22. Rechtman E, Danis RP, Pratt LM, Harris A. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Ophthalmol. 2004. 88:344–347.23. Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005. 112:301–304.24. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000. 20:244–250.25. Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006. 141:149–156.26. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007. 143:566–583.27. Benjamin LE, Golijanin D, Itin A, et al. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999. 103:159–165.28. Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol. 2006. 124:1532–1542.29. Smith BT, Dhalla MS, Shah GK, et al. Intravitreal injection of bevacizumab combined with verteporfin photodynamic therapy for choroidal neovascularization in age-related macular degeneration. Retina. 2008. 28:675–681.30. Isola V, Pece A, Parodi MB. Choroidal ischemia after photodynamic therapy with verteporfin for choroidal neovascularization. Am J Ophthalmol. 2006. 142:680–683.31. Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina. 2007. 27:133–140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Photodynamic Therapy and Intravitreal Bevacizumab Injection for Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy
- Bevacizumab Monotherapy Versus Combined Therapy with Photodynamic Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration
- Effect of Photodynamic Therapy and Intravitreal Triamcinolone Acetonide on Choroidal Neovascularization in Age-related Macular Degeneration
- Treatment of Exudative Age-Related Macular Degeneration
- Effects and Prognostic Factors of Intravitreal Bevacizumab Injection on Choroidal Neovascularization from Age-Related Macular Degeneration