Korean J Ophthalmol.
2010 Aug;24(4):230-236. 10.3341/kjo.2010.24.4.230.
The Effect of Bevacizumab on Corneal Neovascularization in Rabbits
- Affiliations
-
- 1Department of Ophthalmology, St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea. eyedoc@catholic.ac.kr
- KMID: 949061
- DOI: http://doi.org/10.3341/kjo.2010.24.4.230
Abstract
- PURPOSE
To determine the efficacy of topical application and subconjunctival injection of bevacizumab in the treatment of corneal neovascularization.
METHODS
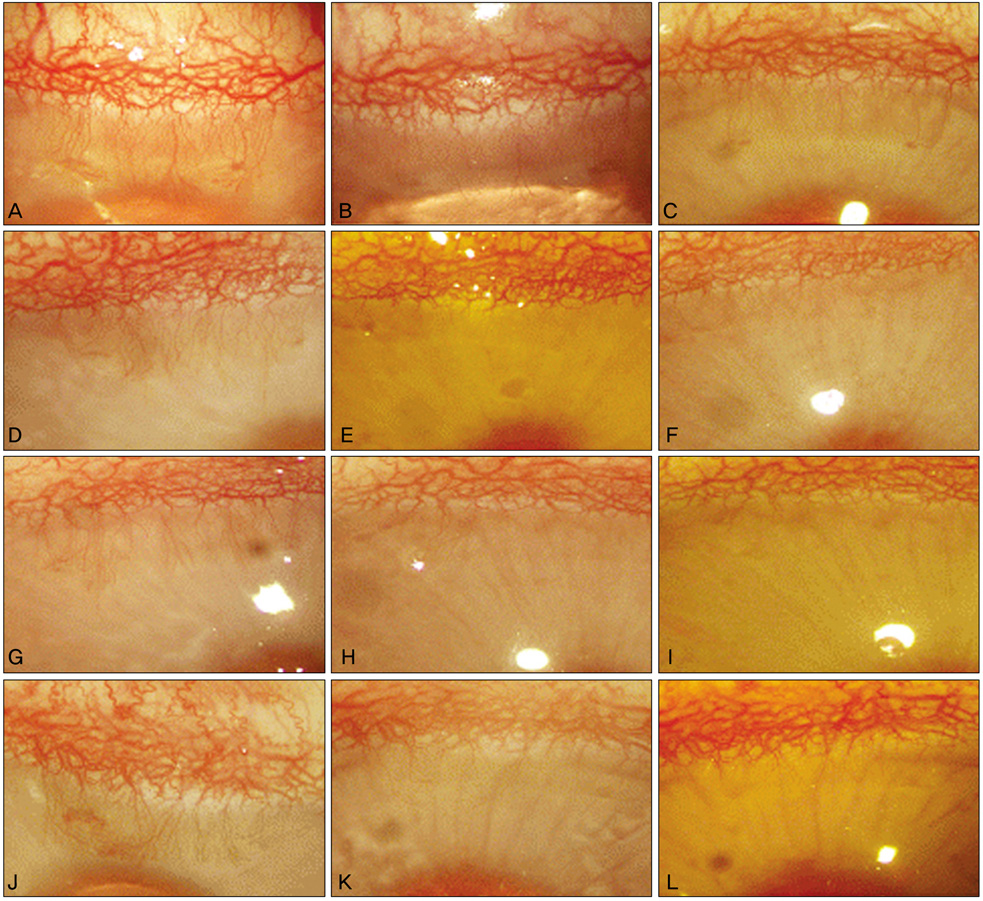
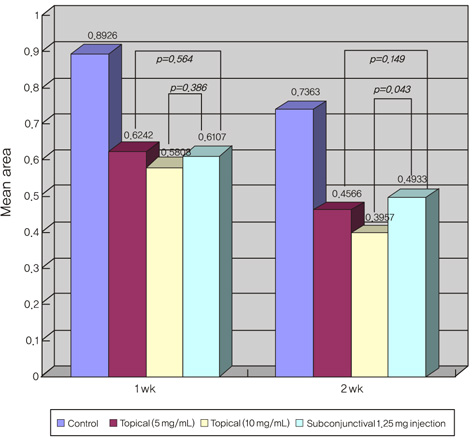
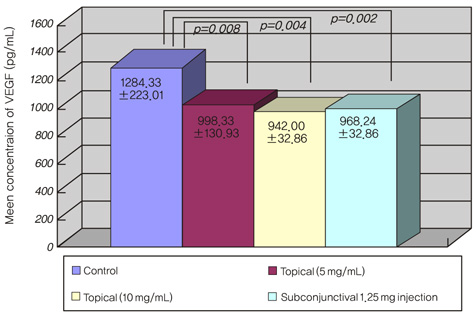
Corneal neovascularization was induced with a silk suture of the corneal stroma in 12 rabbits (24 eyes). One week after suturing, four rabbits were treated with topical bevacizumab at 5 mg/mL (group A) and another four rabbits were treated with topical bevacizumab 10 mg/mL (group B) in the right eyes twice a day for two weeks. A subconjunctival injection of bevacizumab 1.25 mg/mL was done in the right eyes of four rabbits (group C). All of the left eyes (12 eyes) were used as controls. The area of corneal neovascularization was measured after one and two weeks, and the concentration of vascular endothelial growth factor (VEGF) in corneal tissue was measured after two weeks.
RESULTS
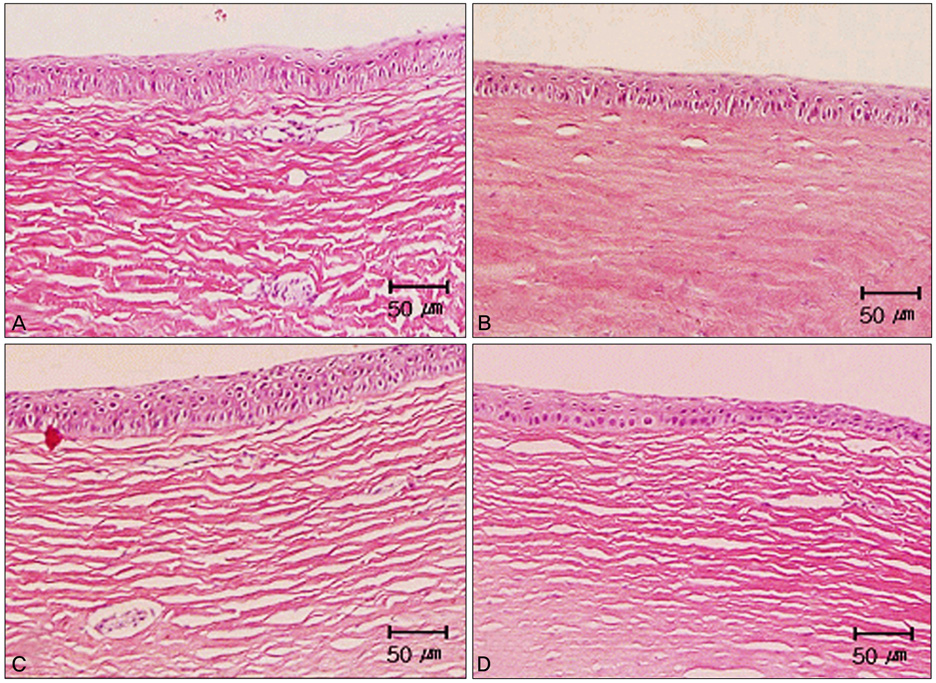
The neovascularized area was smaller in all treated groups than in the control group (p<0.001). Upon analysis of the neovascularized area, there was no significant difference between groups A and B. However, the mean neovascularized area of group B was significantly smaller than that of group C after two weeks of treatment (p=0.043). The histologic examination revealed fewer new corneal vessels in all treated groups than the control group. The concentration of VEGF was significantly lower in all treated groups compared to the control group (p<0.01), but no difference was shown between treated groups.
CONCLUSIONS
Topical and subconjunctival bevacizumab application may be useful in the treatment of corneal neovascularization and further study is necessary.
Keyword
MeSH Terms
-
Angiogenesis Inhibitors/*administration & dosage
Animals
Antibodies, Monoclonal/*administration & dosage
Cornea/metabolism/*pathology
Corneal Neovascularization/*drug therapy/metabolism/pathology
Disease Models, Animal
Dose-Response Relationship, Drug
Drug Administration Schedule
Female
Follow-Up Studies
Male
Ophthalmic Solutions
Rabbits
Treatment Outcome
Vascular Endothelial Growth Factor A/antagonists & inhibitors/metabolism
Figure
Reference
-
1. Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996. 37:2485–2494.2. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998. 43:245–269.3. Riazi-Esfahani M, Peyman GA, Aydin E, et al. Prevention of corneal neovascularization: evaluation of various commercially available compounds in an experimental rat model. Cornea. 2006. 25:801–805.4. Ambati BK, Joussen AM, Ambati J, et al. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002. 120:1063–1068.5. Shao C, Sima J, Zhang SX, et al. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci. 2004. 45:1758–1762.6. Phillips K, Arffa R, Cintron C, et al. Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration, and neovascularization. Arch Ophthalmol. 1983. 101:640–643.7. Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1999. 237:920–927.8. Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006. 244:205–209.9. Mendelsohn AD, Stock EL, Lo GG, Schneck GL. Laser photocoagulation of feeder vessels in lipid keratopathy. Ophthalmic Surg. 1986. 17:502–508.10. Primbs GB, Casey R, Wamser K, et al. Photodynamic therapy for corneal neovascularization. Ophthalmic Surg Lasers. 1998. 29:832–838.11. Nah HJ, Yoon KC, Im WB, et al. Animal study of photodynamic therapy with verteporfin in corneal neovascularization. J Korean Ophthalmol Soc. 2005. 46:707–715.12. Phillips GD, Stone AM, Jones BD, et al. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994. 8:961–965.13. Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000. 19:526–533.14. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000. 41:2514–2522.15. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an antivascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997. 57:4593–4599.16. Emerson MV, Lauer AK, Flaxel CJ, et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007. 27:439–444.17. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363.e5–372.e5.18. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007. 114:743–750.19. Woo KJ, Lee K, Choi DG, Choi MY. The effect of subconjunctival injection of bevacizumab after resection of muscle in rabbit models. J Korean Ophthalmol Soc. 2010. 51:423–429.20. Kang S, Chung SK. The effect of subconjuctival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea. 2010. 29:192–196.21. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001. 12:242–249.22. Benelli U, Ross JR, Nardi M, Klintworth GK. Corneal neovascularization induced by xenografts or chemical cautery: inhibition by cyclosporin A. Invest Ophthalmol Vis Sci. 1997. 38:274–282.23. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000. 343:86–93.24. Yoon KC, Im SK, Oh HJ, Park YG. Two cases of photodynamic therapy with verteporfin in patients with corneal neovascularization. J Korean Ophthalmol Soc. 2006. 47:13–18.25. Jun EJ, Rho YJ, Kim YH, Chung SK. The effect of photodynamic therapy with verteporfin retreatment on corneal neovascularization in rabbits. J Korean Ophthalmol Soc. 2008. 49:1515–1524.26. Bjorndahl MA, Cao R, Burton JB, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005. 65:9261–9268.27. Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004. 18:1111–1113.28. Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004. 7:335–345.29. Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007. 16:437–439.30. Yoeruek E, Spitzer MS, Tatar O, et al. Safety profile of bevacizumab on cultured human corneal cells. Cornea. 2007. 26:977–982.31. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007. 99:1232–1239.32. Quiroz-Mercado H, Ustariz-Gonzalez O, Martinez-Castellanos MA, et al. Our experience after 1765 intravitreal injections of bevacizumab: the importance of being part of a developing story. Semin Ophthalmol. 2007. 22:109–125.33. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007. 48:2545–2552.34. Manzano RP, Peyman GA, Khan P, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol. 2007. 91:804–807.35. Erdurmus M, Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2007. 245:1577–1579.36. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005. 46:726–733.37. Vinores SA. Pegaptanib in the treatment of wet, age-related macular degeneration. Int J Nanomedicine. 2006. 1:263–268.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Cartilage on Corneal Neovascularization
- The Effects of a Subtenoncapsular Injection of Bevacizumab for Ocular Surface Disease With Corneal Neovascularization
- Bi-weekly Subconjunctival Injection of Bevacizumab for Corneal Neovascularization after Burn Injury
- Ranibizumab Injection for Corneal Neovascularization Refractory to Bevacizumab Treatment
- The Effect of Photodynamic Therapy with Verteporfin Retreatment on Corneal Neovascularization in Rabbits