Immune Netw.
2018 Feb;18(1):e12. 10.4110/in.2018.18.e12.
Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 34134, Korea. young@cnu.ac.kr
- 2Institute of Biotechnology, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2404894
- DOI: http://doi.org/10.4110/in.2018.18.e12
Abstract
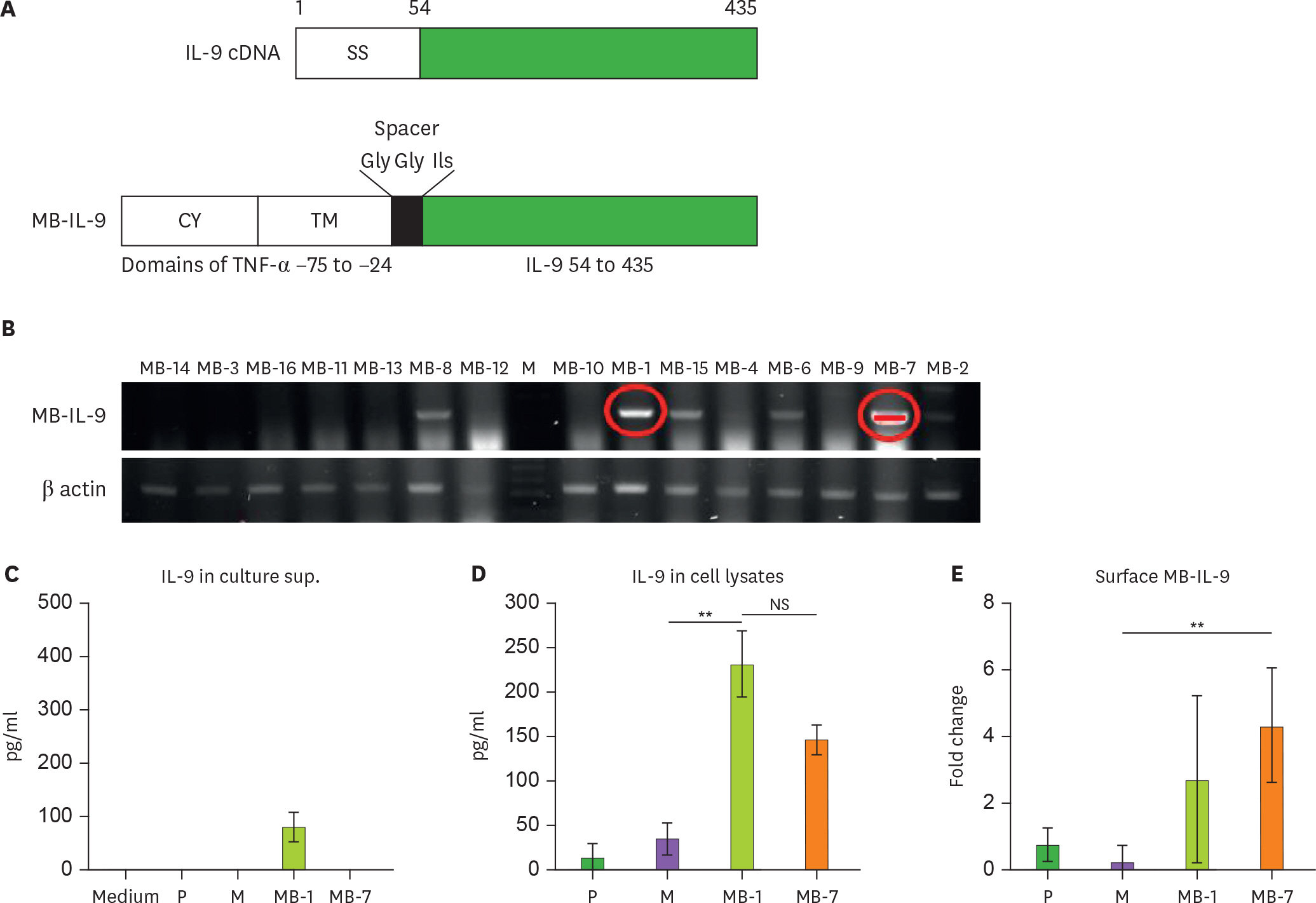
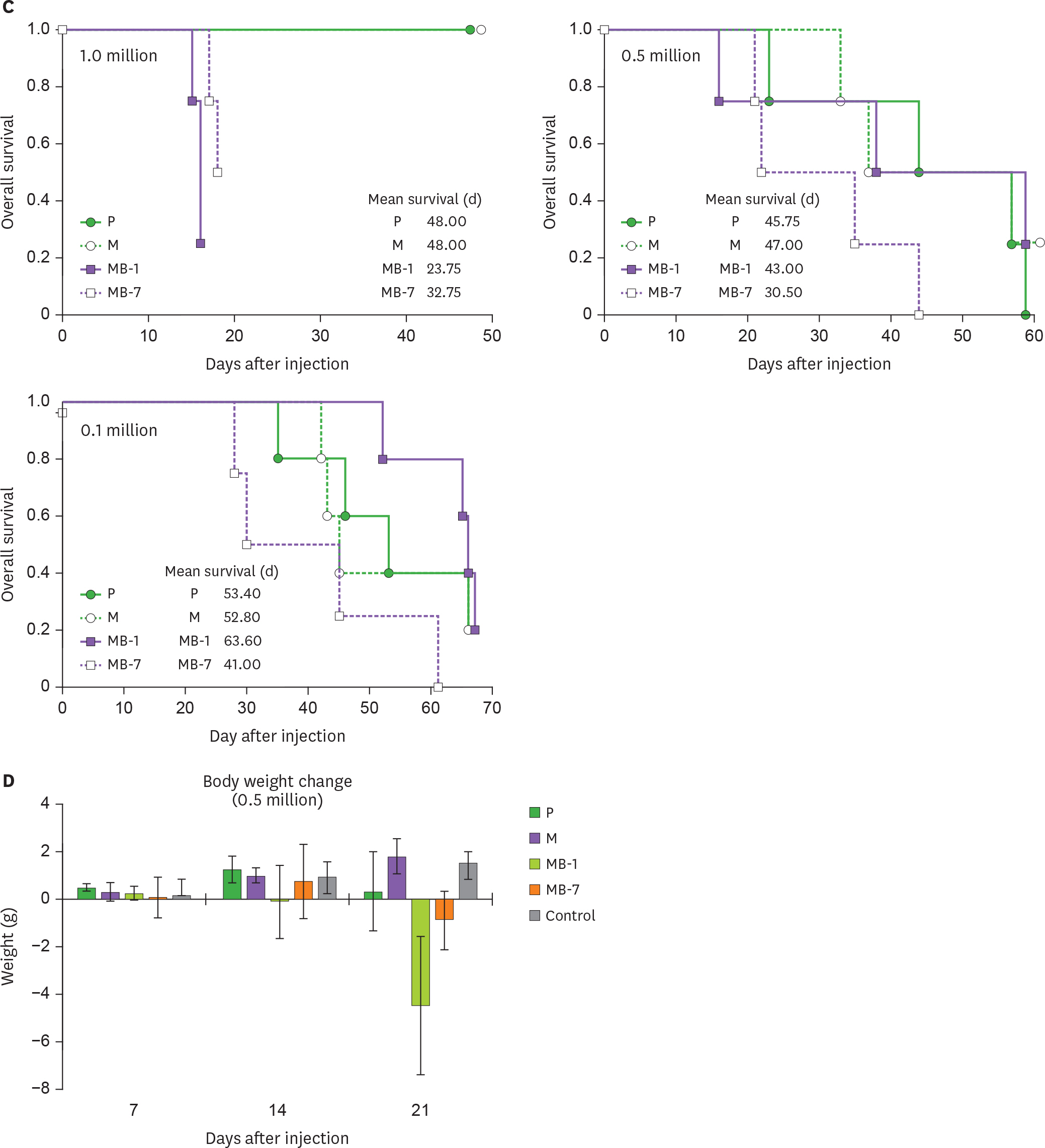
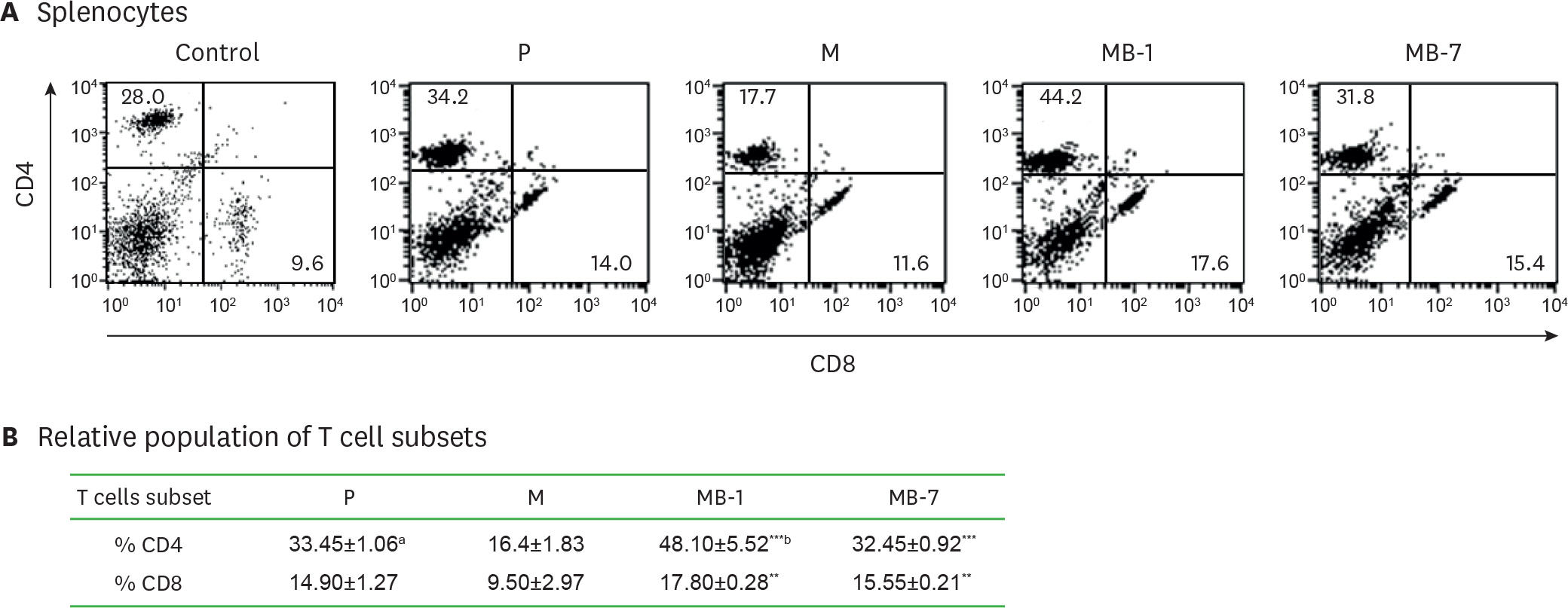
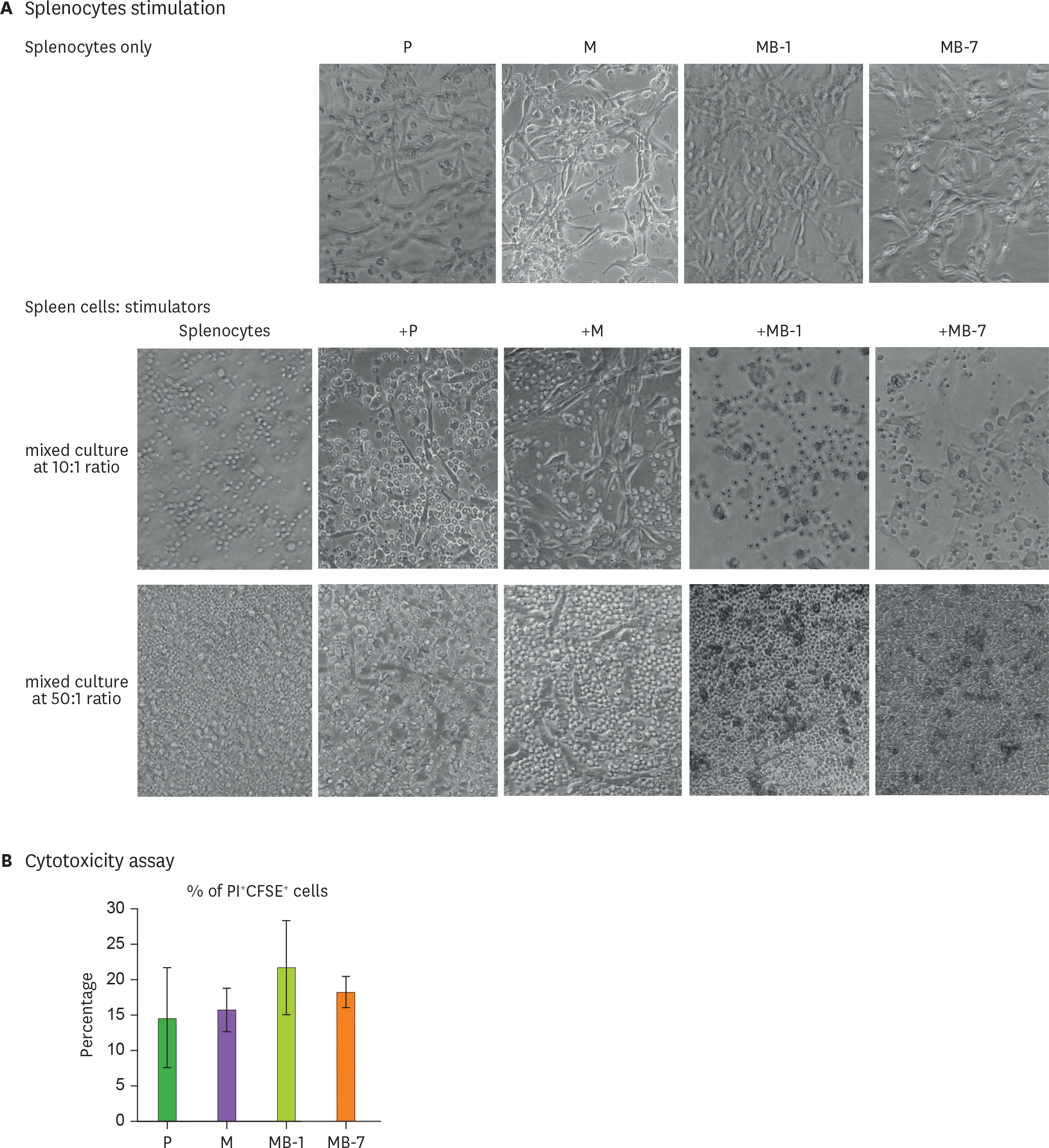
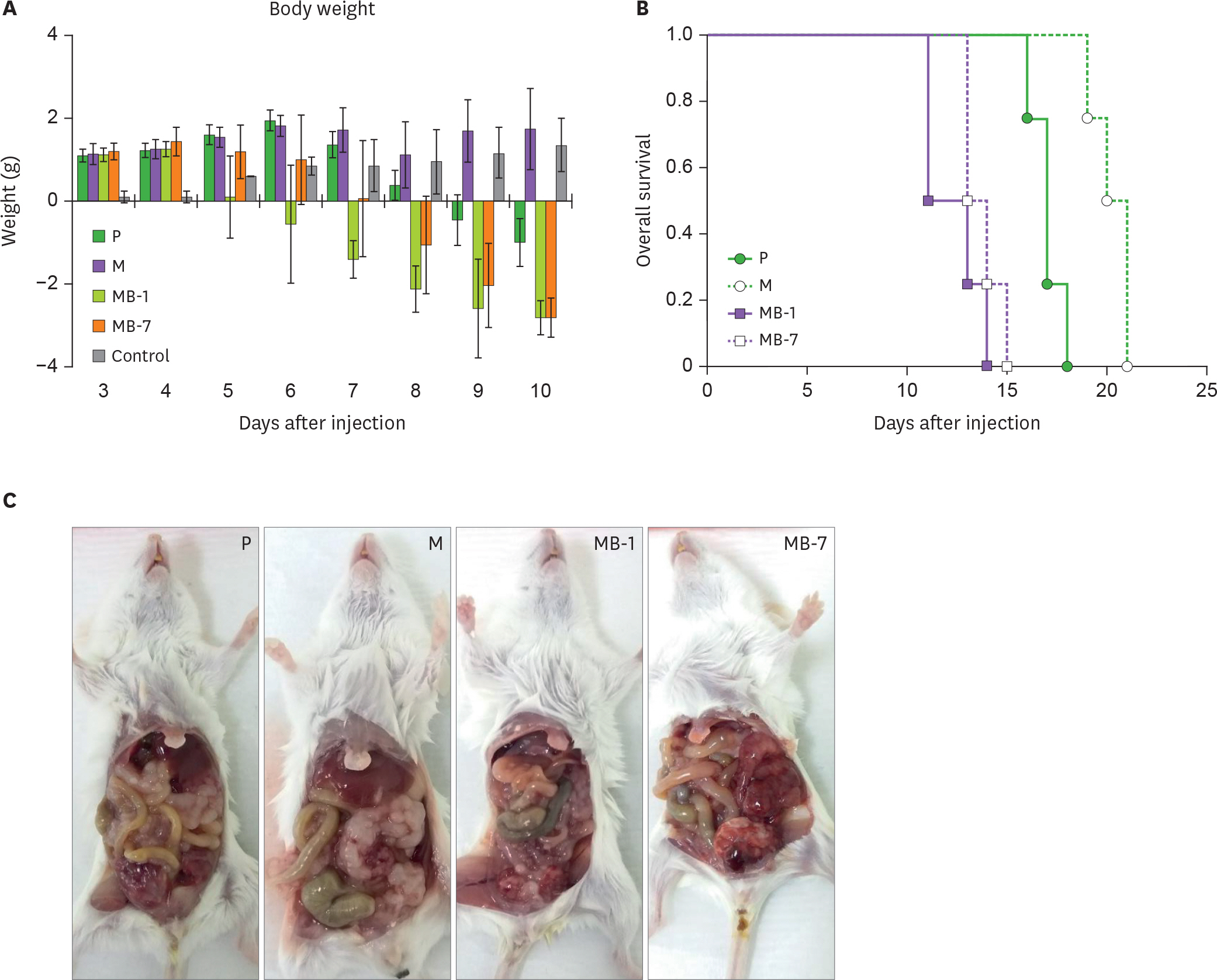
- IL-9 is a known T cell growth factor with pleiotropic immunological functions, especially in parasite infection and colitis. However, its role in tumor growth is controversial. In this study, we generated tumor clones expressing the membrane-bound form of IL-9 (MB-IL-9) and investigated their influences on immune system. MB-IL-9 tumor clones showed reduced tumorigenicity but shortened survival accompanied with severe body weight loss in mice. MB-IL-9 expression on tumor cells had no effect on cell proliferation or major histocompatibility complex class I expression in vitro. MB-IL-9 tumor clones were effective in amplifying CD4⺠and CD8⺠T cells and increasing cytotoxic activity against CT26 cells in vivo. We also observed a prominent reduction in body weights and survival period of mice injected intraperitoneally with MB-IL-9 clones compared with control groups. Ratios of IL-17 to interferon (IFN)-γ in serum level and tumor mass were higher in mice implanted with MB-IL-9 tumor clones than those observed in mice implanted with control cells. These results indicate that the ectopic expression of the MB-IL-9 on tumor cells exerts an immune-stimulatory effect with toxicity. To exploit its benefits as a tumor vaccine, a strategy to control the toxicity of MB-IL-9 tumor clones should be developed.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988; 85:6934–6938.
Article2. Schmitt E, Bopp T. Amazing IL-9: revealing a new function for an “old” cytokine. J Clin Invest. 2012; 122:3857–3859.
Article3. Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994; 9:1327–1332.4. Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms'the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008; 9:1341–1346.5. Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3 (-) effector T cells. Nat Immunol. 2008; 9:1347–1355.6. Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010; 185:46–54.7. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442:997–1002.
Article8. Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 2012; 1247:56–68.
Article9. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534.
Article10. Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(h)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007; 8:958–966.
Article11. Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012; 18:1248–1253.
Article12. Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, Bae EA, Lee GE, Jeon H, Cho J, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein costimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015; 21:1010–1017.
Article13. Parrot T, Allard M, Oger R, Benlalam H, Raingeard de la Bletiere D, Coutolleau A, Preisser L, Desfrancois J, Khammari A, Dreno B, et al. IL-9 promotes the survival and function of human melanoma-infiltrating CD4 (+) CD8 (+) double-positive T cells. Eur J Immunol. 2016; 46:1770–1782.14. Fang Y, Chen X, Bai Q, Qin C, Mohamud AO, Zhu Z, Ball TW, Ruth CM, Newcomer DR, Herrick EJ, et al. IL-9 inhibits HTB-72 melanoma cell growth through upregulation of p21 and TRAIL. J Surg Oncol. 2015; 111:969–974.
Article15. Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, Qian J, Yi Q. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci U S A. 2014; 111:2265–2270.16. Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012; 122:4160–4171.17. Liu JQ, Li XY, Yu HQ, Yang G, Liu ZQ, Geng XR, Wang S, Mo LH, Zeng L, Zhao M, et al. Tumor-specific Th2 responses inhibit growth of CT26 colon-cancer cells in mice via converting intratumor regulatory T cells to Th9 cells. Sci Rep. 2015; 5:10665.
Article18. Rivera Vargas T, Humblin E, Végran F, Ghiringhelli F, Apetoh L. TH9 cells in anti-tumor immunity. Semin Immunopathol. 2017; 39:39–46.
Article19. Huang Y, Cao Y, Zhang S, Gao F. Association between low expression levels of interleukin-9 and colon cancer progression. Exp Ther Med. 2015; 10:942–946.
Article20. Zhao Y, Chu X, Chen J, Wang Y, Gao S, Jiang Y, Zhu X, Tan G, Zhao W, Yi H, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun. 2016; 7:12368.
Article21. Nakatsukasa H, Zhang D, Maruyama T, Chen H, Cui K, Ishikawa M, Deng L, Zanvit P, Tu E, Jin W, et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4 (+) T cells. Nat Immunol. 2015; 16:1077–1084.
Article22. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005; 167:835–848.23. Abdul-Wahid A, Cydzik M, Prodeus A, Alwash M, Stanojcic M, Thompson M, Huang EH, Shively JE, Gray-Owen SD, Gariepy J. Induction of antigen-specific TH 9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int J Cancer. 2016; 139:841–853.24. Hoelzinger DB, Dominguez AL, Cohen PA, Gendler SJ. Inhibition of adaptive immunity by IL9 can be disrupted to achieve rapid T-cell sensitization and rejection of progressive tumor challenges. Cancer Res. 2014; 74:6845–6855.
Article25. Lv X, Feng L, Ge X, Lu K, Wang X. Interleukin-9 promotes cell survival and drug resistance in diffuse large B-cell lymphoma. J Exp Clin Cancer Res. 2016; 35:106.
Article26. Zhang J, Wang WD, Geng QR, Wang L, Chen XQ, Liu CC, Lv Y. Serum levels of interleukin-9 correlate with negative prognostic factors in extranodal NK/T-cell lymphoma. PLoS One. 2014; 9:e94637.
Article27. Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004; 22:207–215.
Article28. Fischer M, Bijman M, Molin D, Cormont F, Uyttenhove C, van Snick J, Sundstrom C, Enblad G, Nilsson G. Increased serum levels of interleukin-9 correlate to negative prognostic factors in Hodgkin's lymphoma. Leukemia. 2003; 17:2513–2516.
Article29. Chen N, Lu K, Li P, Lv X, Wang X. Overexpression of IL-9 induced by STAT6 activation promotes the pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp Pathol. 2014; 7:2319–2323.30. Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009; 106:12885–12890.
Article31. Kim YS. Tumor therapy applying membrane-bound form of cytokines. Immune Netw. 2009; 9:158–168.
Article32. Do Thi VA, Park SM, Lee H, Kim YS. The membrane-bound form of IL-17A promotes the growth and tumorigenicity of colon cancer cells. Mol Cells. 2016; 39:536–542.33. Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). 2010; 49:1215–1228.34. Väyrynen JP, Kantola T, Väyrynen SA, Klintrup K, Bloigu R, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016; 139:112–121.
Article35. Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 2010; 7:182–189.
Article36. Lundy SK, Fox DA. Reduced Fas ligand-expressing splenic CD5+ B lymphocytes in severe collagen-induced arthritis. Arthritis Res Ther. 2009; 11:R128.
Article37. Chang MR, Lee WH, Choi JW, Park SO, Paik SG, Kim YS. Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2. Exp Mol Med. 2005; 37:240–249.
Article38. Kim HJ, Park SM, Lee H, Kim YS. Membrane-bound p35 Subunit of IL-12 on tumor cells is functionally equivalent to membrane-bound heterodimeric single chain IL-12 for induction of anti-tumor immunity. Immune Netw. 2016; 16:305–310.
Article39. Kim YS, Sonn CH, Paik SG, Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000; 7:837–843.
Article40. Lim HY, Ju HY, Chung HY, Kim YS. Antitumor effects of a tumor cell vaccine expressing a membrane-bound form of the IL-12 p35 subunit. Cancer Biol Ther. 2010; 10:336–343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane-bound p35 Subunit of IL-12 on Tumor Cells is Functionally Equivalent to Membrane-bound Heterodimeric Single Chain IL-12 for Induction of Anti-tumor Immunity
- Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- Tumor Therapy Applying Membrane-bound Form of Cytokines
- Mechanism of Differential Ag-specific Immune Induction by Different Tumor Cell Lysate Pulsed DC
- Stimulatory Effect of IL-10 on Antitumor Cytolytic Activity of Murine Spleen Cells