Korean J Radiol.
2015 Dec;16(6):1353-1363. 10.3348/kjr.2015.16.6.1353.
Subtraction MR Venography Acquired from Time-Resolved Contrast-Enhanced MR Angiography: Comparison with Phase-Contrast MR Venography and Single-Phase Contrast-Enhanced MR Venography
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. bkim.neurorad@gmail.com
- 2Department of Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon 16247, Korea.
- KMID: 2344292
- DOI: http://doi.org/10.3348/kjr.2015.16.6.1353
Abstract
OBJECTIVE
To evaluate the image characteristics of subtraction magnetic resonance venography (SMRV) from time-resolved contrast-enhanced MR angiography (TRMRA) compared with phase-contrast MR venography (PCMRV) and single-phase contrast-enhanced MR venography (CEMRV).
MATERIALS AND METHODS
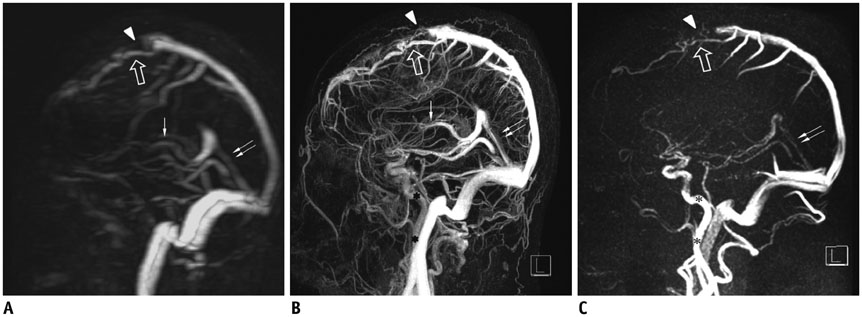
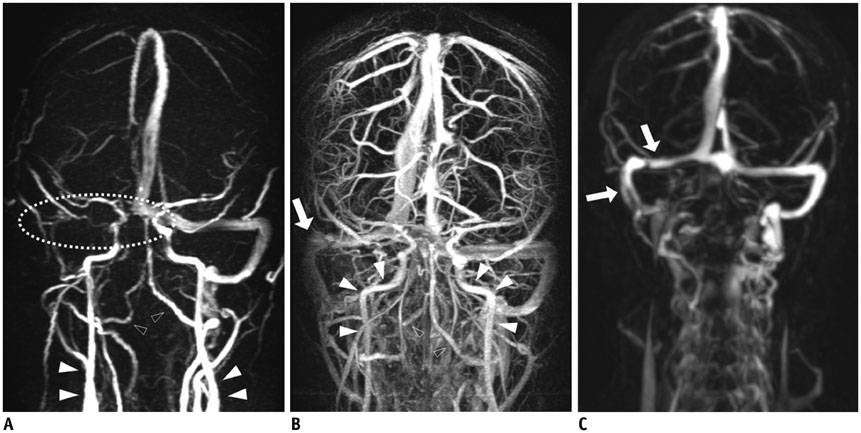
Twenty-one patients who underwent brain MR venography (MRV) using standard protocols (PCMRV, CEMRV, and TRMRA) were included. SMRV was made by subtracting the arterial phase data from the venous phase data in TRMRA. Co-registration and subtraction of the two volume data was done using commercially available software. Image quality and the degree of arterial contamination of the three MRVs were compared. In the three MRVs, 19 pre-defined venous structures (14 dural sinuses and 5 cerebral veins) were evaluated. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of the three MRVs were also compared.
RESULTS
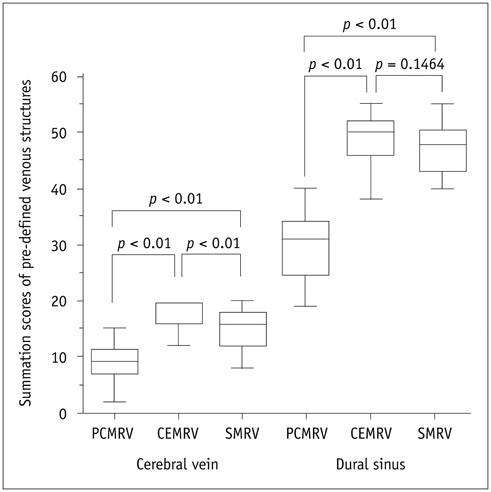
Single-phase contrast-enhanced MR venography showed better image quality (median score 4 in both reviewers) than did the other two MRVs (p < 0.001), whereas SMRV (median score 3 in both reviewers) and PCMRV (median score 3 in both reviewers) had similar image quality (p ≥ 0.951). SMRV (median score 0 in both reviewers) suppressed arterial signal better than did the other MRVs (median score 1 in CEMRV, median score 2 in PCMRV, both reviewers) (p < 0.001). The dural sinus score of SMRV (median and interquartile range [IQR] 48, 43-50 for reviewer 1, 47, 43-49 for reviewer 2) was significantly higher than for PCMRV (median and IQR 31, 25-34 for reviewer 1, 30, 23-32 for reviewer 2) (p < 0.01) and did not differ from that of CEMRV (median and IQR 50, 47-52 for reviewer 1, 49, 45-51 for reviewer 2) (p = 0.146 in reviewer 1 and 0.123 in reviewer 2). The SNR and CNR of SMRV (median and IQR 104.5, 83.1-121.2 and 104.1, 74.9-120.5, respectively) were between those of CEMRV (median and IQR 150.3, 111-182.6 and 148.4, 108-178.2) and PCMRV (median and IQR 59.4, 49.2-74.9 and 53.6, 43.8-69.2).
CONCLUSION
Subtraction magnetic resonance venography is a promising MRV method, with acceptable image quality and good arterial suppression.
Keyword
MeSH Terms
Figure
Reference
-
1. Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000; 21:74–78.2. Liauw L, van Buchem MA, Spilt A, de Bruïne FT, van den Berg R, Hermans J, et al. MR angiography of the intracranial venous system. Radiology. 2000; 214:678–682.3. Dolic K, Siddiqui AH, Karmon Y, Marr K, Zivadinov R. The role of noninvasive and invasive diagnostic imaging techniques for detection of extra-cranial venous system anomalies and developmental variants. BMC Med. 2013; 11:155.4. Meckel S, Reisinger C, Bremerich J, Damm D, Wolbers M, Engelter S, et al. Cerebral venous thrombosis: diagnostic accuracy of combined, dynamic and static, contrast-enhanced 4D MR venography. AJNR Am J Neuroradiol. 2010; 31:527–535.5. Zivadinov R, Lopez-Soriano A, Weinstock-Guttman B, Schirda CV, Magnano CR, Dolic K, et al. Use of MR venography for characterization of the extracranial venous system in patients with multiple sclerosis and healthy control subjects. Radiology. 2011; 258:562–570.6. Meckel S, Glücker TM, Kretzschmar M, Scheffler K, Radü EW, Wetzel SG. Display of dural sinuses with time-resolved, contrast-enhanced three-dimensional MR venography. Cerebrovasc Dis. 2008; 25:217–224.7. Yiğit H, Turan A, Ergün E, Kosşar P, Kosşar U. Time-resolved MR angiography of the intracranial venous system: an alternative MR venography technique. Eur Radiol. 2012; 22:980–989.8. McTaggart RA, Fischbein NJ, Elkins CJ, Hsiao A, Cutalo MJ, Rosenberg J, et al. Extracranial venous drainage patterns in patients with multiple sclerosis and healthy controls. AJNR Am J Neuroradiol. 2012; 33:1615–1620.9. Rahman MT, Sethi SK, Utriainen DT, Hewett JJ, Haacke EM. A comparative study of magnetic resonance venography techniques for the evaluation of the internal jugular veins in multiple sclerosis patients. Magn Reson Imaging. 2013; 31:1668–1676.10. Lim RP, Shapiro M, Wang EY, Law M, Babb JS, Rueff LE, et al. 3D time-resolved MR angiography (MRA) of the carotid arteries with time-resolved imaging with stochastic trajectories: comparison with 3D contrast-enhanced Bolus-Chase MRA and 3D time-of-flight MRA. AJNR Am J Neuroradiol. 2008; 29:1847–1854.11. Lee YJ, Laub G, Jung SL, Yoo WJ, Kim YJ, Ahn KJ, et al. Low-dose 3D time-resolved magnetic resonance angiography (MRA) of the supraaortic arteries: correlation with high spatial resolution 3D contrast-enhanced MRA. J Magn Reson Imaging. 2011; 33:71–76.12. Haider CR, Hu HH, Campeau NG, Huston J 3rd, Riederer SJ. 3D high temporal and spatial resolution contrast-enhanced MR angiography of the whole brain. Magn Reson Med. 2008; 60:749–760.13. Nael K, Fenchel M, Salamon N, Duckwiler GR, Laub G, Finn JP, et al. Three-dimensional cerebral contrast-enhanced magnetic resonance venography at 3.0 Tesla: initial results using highly accelerated parallel acquisition. Invest Radiol. 2006; 41:763–768.14. Lee YJ, Kim BS, Koo JS, Kim BY, Jang J, Choi HS, et al. Supra-aortic low-dose contrast-enhanced time-resolved magnetic resonance (MR) angiography at 3 T: comparison with time-of-flight MR angiography and high-resolution contrast-enhanced MR angiography. Acta Radiol. 2015; 56:673–680.15. Ruhl KM, Katoh M, Langer S, Mommertz G, Guenther RW, Niendorf T, et al. Time-resolved 3D MR angiography of the foot at 3 T in patients with peripheral arterial disease. AJR Am J Roentgenol. 2008; 190:W360–W364.16. Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: methods and applications. NMR Biomed. 2006; 19:325–341.17. Zivadinov R, Galeotti R, Hojnacki D, Menegatti E, Dwyer MG, Schirda C, et al. Value of MR venography for detection of internal jugular vein anomalies in multiple sclerosis: a pilot longitudinal study. AJNR Am J Neuroradiol. 2011; 32:938–946.18. Fera F, Bono F, Messina D, Gallo O, Lanza PL, Auteri W, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol. 2005; 252:1021–1025.19. Boetes C, Barentsz JO, Mus RD, van der Sluis RF, van Erning LJ, Hendriks JH, et al. MR characterization of suspicious breast lesions with a gadolinium-enhanced TurboFLASH subtraction technique. Radiology. 1994; 193:777–781.20. Anxionnat R, Bracard S, Ducrocq X, Trousset Y, Launay L, Kerrien E, et al. Intracranial aneurysms: clinical value of 3D digital subtraction angiography in the therapeutic decision and endovascular treatment. Radiology. 2001; 218:799–808.21. Sugahara T, Korogi Y, Nakashima K, Hamatake S, Honda S, Takahashi M. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol. 2002; 23:1545–1552.22. Uemura M, Miyagawa M, Yasuhara Y, Murakami T, Ikura H, Sakamoto K, et al. Clinical evaluation of pulmonary nodules with dual-exposure dual-energy subtraction chest radiography. Radiat Med. 2005; 23:391–397.23. Chilcote WA, Modic MT, Pavlicek WA, Little JR, Furlan AJ, Duchesneau PM, et al. Digital subtraction angiography of the carotid arteries: a comparative study in 100 patients. Radiology. 1981; 139:287–295.24. Acar M, Degirmenci B, Yucel A, Albayrak R, Haktanir A. An evaluation of internal carotid artery and cerebral blood flow volume using color duplex sonography in patients with vertebral artery hypoplasia. Eur J Radiol. 2005; 53:450–453.25. Ford MD, Alperin N, Lee SH, Holdsworth DW, Steinman DA. Characterization of volumetric flow rate waveforms in the normal internal carotid and vertebral arteries. Physiol Meas. 2005; 26:477–488.26. Jung H, Sung K, Nayak KS, Kim EY, Ye JC. k-t FOCUSS: a general compressed sensing framework for high resolution dynamic MRI. Magn Reson Med. 2009; 61:103–116.27. Rapacchi S, Natsuaki Y, Plotnik A, Gabriel S, Laub G, Finn JP, et al. Reducing view-sharing using compressed sensing in time-resolved contrast-enhanced magnetic resonance angiography. Magn Reson Med. 2015; 74:474–481.28. Andeweg J. The anatomy of collateral venous flow from the brain and its value in aetiological interpretation of intracranial pathology. Neuroradiology. 1996; 38:621–628.29. Kudo K, Terae S, Ishii A, Omatsu T, Asano T, Tha KK, et al. Physiologic change in flow velocity and direction of dural venous sinuses with respiration: MR venography and flow analysis. AJNR Am J Neuroradiol. 2004; 25:551–557.30. Schaller B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Brain Res Rev. 2004; 46:243–260.31. Kopelman M, Glik A, Greenberg S, Shelef I. Intracranial nonjugular venous pathways: a possible compensatory drainage mechanism. AJNR Am J Neuroradiol. 2013; 34:1348–1352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Contrast Enhanced Rapid MR Sequence(True FISP) in Deep Vein Thrombosis
- The Usefulness of Three-Dimensional Gadolinium-Enhanced MR Venography for the Evaluation of Varices in Lower Extremities
- Contrast Enhanced Cerebral MR Venography: Comparison between Arterial and Venous Triggering Methods
- Gadolinium-Enhanced 3D Fast Imaging Steady-State Procession(FISP) MR Venography in the Deep Vein Thrombosis of Low Extremities
- Ultrafast Contrast-Enhanced MR Angiography of the Carotid Artery: Time Optimization for Discrimination of theArterial from the Venous Phase