Nutr Res Pract.
2016 Feb;10(1):11-18. 10.4162/nrp.2016.10.1.11.
D-Xylose as a sugar complement regulates blood glucose levels by suppressing phosphoenolpyruvate carboxylase (PEPCK) in streptozotocin-nicotinamide-induced diabetic rats and by enhancing glucose uptake in vitro
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52 Ewhayeodae-gil, Seodaemun-gu, Seoul 03760, Korea. yuri.kim@ewha.ac.kr
- 2R&D center, TS Corporation, Incheon 400-201, Korea.
- 3Department of Food Science & Technology, BK21 Plus Team, and Carbohydrate Bioproduct Research Center, Sejong University, Seoul 05006, Korea.
- KMID: 2313895
- DOI: http://doi.org/10.4162/nrp.2016.10.1.11
Abstract
- BACKGROUND/OBJECTIVES
Type 2 diabetes (T2D) is more frequently diagnosed and is characterized by hyperglycemia and insulin resistance. D-Xylose, a sucrase inhibitor, may be useful as a functional sugar complement to inhibit increases in blood glucose levels. The objective of this study was to investigate the anti-diabetic effects of D-xylose both in vitro and stretpozotocin (STZ)-nicotinamide (NA)-induced models in vivo.
MATERIALS/METHODS
Wistar rats were divided into the following groups: (i) normal control; (ii) diabetic control; (iii) diabetic rats supplemented with a diet where 5% of the total sucrose content in the diet was replaced with D-xylose; and (iv) diabetic rats supplemented with a diet where 10% of the total sucrose content in the diet was replaced with D-xylose. These groups were maintained for two weeks. The effects of D-xylose on blood glucose levels were examined using oral glucose tolerance test, insulin secretion assays, histology of liver and pancreas tissues, and analysis of phosphoenolpyruvate carboxylase (PEPCK) expression in liver tissues of a STZ-NA-induced experimental rat model. Levels of glucose uptake and insulin secretion by differentiated C2C12 muscle cells and INS-1 pancreatic beta-cells were analyzed.
RESULTS
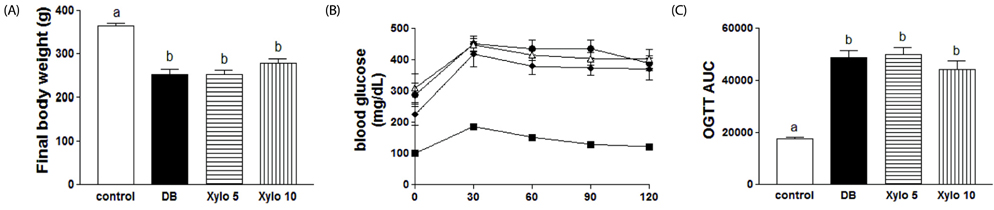
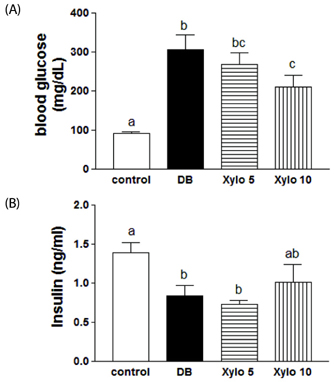
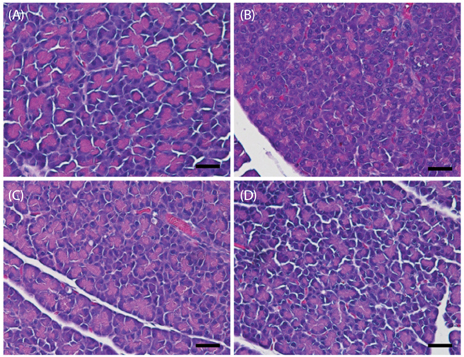
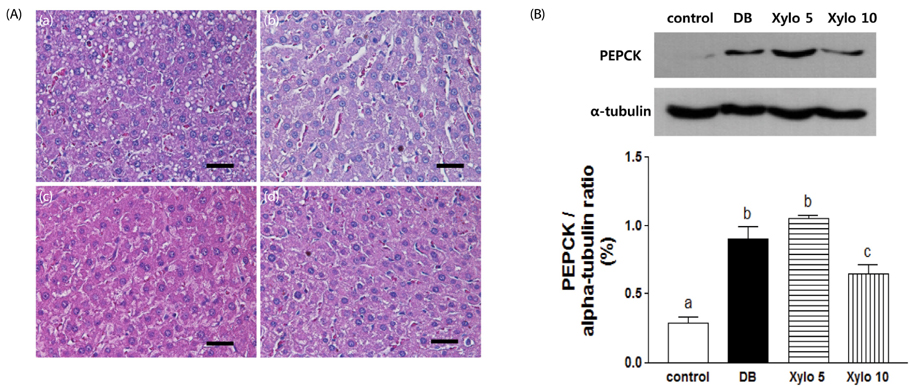
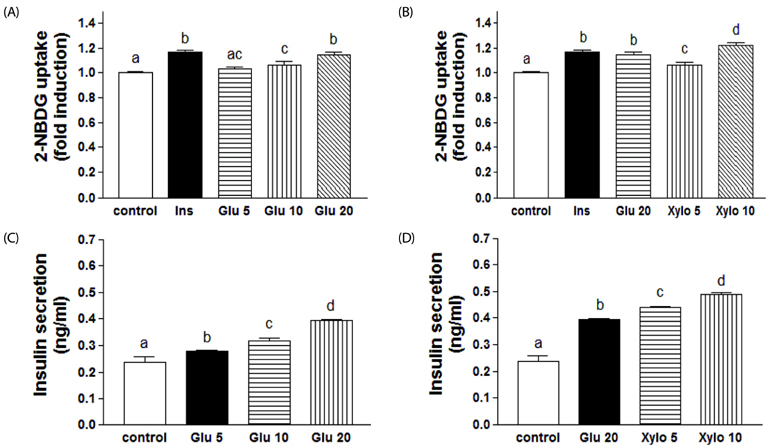
In vivo, D-xylose supplementation significantly reduced fasting serum glucose levels (P < 0.05), it slightly reduced the area under the glucose curve, and increased insulin levels compared to the diabetic controls. D-Xylose supplementation enhanced the regeneration of pancreas tissue and improved the arrangement of hepatocytes compared to the diabetic controls. Lower levels of PEPCK were detected in the liver tissues of D-xylose-supplemented rats (P < 0.05). In vitro, both 2-NBDG uptake by C2C12 cells and insulin secretion by INS-1 cells were increased with D-xylose supplementation in a dose-dependent manner compared to treatment with glucose alone.
CONCLUSIONS
In this study, D-xylose exerted anti-diabetic effects in vivo by regulating blood glucose levels via regeneration of damaged pancreas and liver tissues and regulation of PEPCK, a key rate-limiting enzyme in the process of gluconeogenesis. In vitro, D-xylose induced the uptake of glucose by muscle cells and the secretion of insulin cells by beta-cells. These mechanistic insights will facilitate the development of highly effective strategy for T2D.
Keyword
MeSH Terms
-
Animals
Blood Glucose*
Complement System Proteins*
Diet
Fasting
Gluconeogenesis
Glucose Tolerance Test
Glucose*
Hepatocytes
Hyperglycemia
Insulin
Insulin Resistance
Liver
Models, Animal
Muscle Cells
Pancreas
Phosphoenolpyruvate Carboxylase*
Phosphoenolpyruvate*
Rats*
Rats, Wistar
Regeneration
Sucrase
Sucrose
Xylose*
Blood Glucose
Complement System Proteins
Glucose
Insulin
Phosphoenolpyruvate
Phosphoenolpyruvate Carboxylase
Sucrase
Sucrose
Xylose
Figure
Reference
-
1. Consensus Development Conference on Insulin Resistance. 5-6 November 1997. American Diabetes Association. Diabetes Care. 1998; 21:310–314.2. Li PB, Lin WL, Wang YG, Peng W, Cai XY, Su WW. Antidiabetic activities of oligosaccharides of Ophiopogonis japonicus in experimental type 2 diabetic rats. Int J Biol Macromol. 2012; 51:749–755.
Article3. Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007; 91:1063–1077. viii
Article4. Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat Characteristics of the experimental model. Exp Biol Med (Maywood). 2012; 237:481–490.
Article5. Turk J, Corbett JA, Ramanadham S, Bohrer A, McDaniel ML. Biochemical evidence for nitric oxide formation from streptozotocin in isolated pancreatic islets. Biochem Biophys Res Commun. 1993; 197:1458–1464.
Article6. Wada R, Yagihashi S. Nitric oxide generation and poly(ADP ribose) polymerase activation precede beta-cell death in rats with a single high-dose injection of streptozotocin. Virchows Arch. 2004; 444:375–382.
Article7. Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009; 14:3446–3485.
Article8. Uchigata Y, Yamamoto H, Kawamura A, Okamoto H. Protection by superoxide dismutase, catalase, and poly(ADP-ribose) synthetase inhibitors against alloxan- and streptozotocin-induced islet DNA strand breaks and against the inhibition of proinsulin synthesis. J Biol Chem. 1982; 257:6084–6088.
Article9. Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998; 47:224–229.
Article10. Morral N. Novel targets and therapeutic strategies for type 2 diabetes. Trends Endocrinol Metab. 2003; 14:169–175.
Article11. McAnuff MA, Omoruyi FO, Morrison EY, Asemota HN. Changes in some liver enzymes in streptozotocin-induced diabetic rats fed sapogenin extract from bitter yam (Dioscorea polygonoides) or commercial diosgenin. West Indian Med J. 2005; 54:97–101.
Article12. Brenner RR, Rimoldi OJ, Lombardo YB, González MS, Bernasconi AM, Chicco A, Basabe JC. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids. 2003; 38:733–742.
Article13. Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009; 89:1037–1042.
Article14. Kafatos A, Codrington CA. Nutrition and diet for healthy lifestyles in Europe: the 'Eurodiet' Project. Public Health Nutr. 1999; 2:327–328.
Article15. Yokozawa T, Kim HY, Cho EJ. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J Agric Food Chem. 2002; 50:5485–5489.
Article16. Bae YJ, Bak YK, Kim B, Kim MS, Lee JH, Sung MK. Coconut-derived D-xylose affects postprandial glucose and insulin responses in healthy individuals. Nutr Res Pract. 2011; 5:533–539.
Article17. Seri K, Sanai K, Matsuo N, Kawakubo K, Xue C, Inoue S. L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals. Metabolism. 1996; 45:1368–1374.
Article18. Gruzman A, Shamni O, Ben Yakir M, Sandovski D, Elgart A, Alpert E, Cohen G, Hoffman A, Katzhendler Y, Cerasi E, Sasson S. Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha. J Med Chem. 2008; 51:8096–8108.
Article19. Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997; 66:581–611.
Article20. Weng Y, Yu L, Cui J, Zhu YR, Guo C, Wei G, Duan JL, Yin Y, Guan Y, Wang YH, Yang ZF, Xi MM, Wen AD. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J Ethnopharmacol. 2014; 152:553–560.
Article21. Ananda PK, Kumarappan CT, Sunil C, Kalaichelvan VK. Effect of Biophytum sensitivum on streptozotocin and nicotinamide-induced diabetic rats. Asian Pac J Trop Biomed. 2012; 2:31–35.
Article22. Park KJ, Jin HS, Park SH, Kim EH, Kim JK. Antihyperglycemia effect of medicinal plants mixture in streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2008; 37:1554–1559.
Article23. Pierre W, Gildas AJ, Ulrich MC, Modeste WN, Benoît NT, Albert K. Hypoglycemic and hypolipidemic effects of Bersama engleriana leaves in nicotinamide/streptozotocin-induced type 2 diabetic rats. BMC Complement Altern Med. 2012; 12:264.
Article24. Cheon H, Cho JM, Kim S, Baek SH, Lee MK, Kim KW, Yu SW, Solinas G, Kim SS, Lee MS. Role of JNK activation in pancreatic beta-cell death by streptozotocin. Mol Cell Endocrinol. 2010; 321:131–137.25. Novelli M, Bonamassa B, Masini M, Funel N, Canistro D, De Tata V, Martano M, Soleti A, Campani D, Paolini M, Masiello P. Persistent correction of hyperglycemia in streptozotocin-nicotinamide-induced diabetic mice by a non-conventional radical scavenger. Naunyn Schmiedebergs Arch Pharmacol. 2010; 382:127–137.
Article26. Saravanan R, Pari L. Succinic acid monoethyl ester, a novel insulinotropic agent: effect on lipid composition and lipid peroxidation in streptozotocin-nicotin-amide induced type 2 diabetic rats. Mol Cell Biochem. 2007; 296:165–176.
Article27. Risbud MV, Bhonde RR. Models of pancreatic regeneration in diabetes. Diabetes Res Clin Pract. 2002; 58:155–165.
Article28. Prasath GS, Pillai SI, Subramanian SP. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur J Pharmacol. 2014; 740:248–254.
Article29. Kuo YC, Chen IY, Chang SC, Wu SC, Hung TM, Lee PH, Shimotohno K, Chang MF. Hepatitis C virus NS5A protein enhances gluconeogenesis through upregulation of Akt-/JNK-PEPCK signalling pathways. Liver Int. 2014; 34:1358–1368.
Article30. Pagliassotti MJ, Shahrokhi KA, Moscarello M. Involvement of liver and skeletal muscle in sucrose-induced insulin resistance: dose-response studies. Am J Physiol. 1994; 266:R1637–R1644.
Article31. Mosseri R, Waner T, Shefi M, Shafrir E, Meyerovitch J. Gluconeogenesis in non-obese diabetic (NOD) mice: in vivo effects of vandadate treatment on hepatic glucose-6-phoshatase and phosphoenolpyruvate carboxykinase. Metabolism. 2000; 49:321–325.
Article32. Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006; 3:403–416.
Article33. Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004; 117:5479–5487.
Article34. Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG Jr, Xiong Y, Daniel KW, Floering L, Collins S. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem. 2005; 280:42731–42737.
Article35. Qiu J, Maekawa K, Kitamura Y, Miyata Y, Tanaka K, Tanaka T, Soga M, Tsuda T, Matsui T. Stimulation of glucose uptake by theasinensins through the AMP-activated protein kinase pathway in rat skeletal muscle cells. Biochem Pharmacol. 2014; 87:344–351.
Article36. Lee BH, Hsu WH, Liao TH, Pan TM. The Monascus metabolite monascin against TNF-α-induced insulin resistance via suppressing PPAR-γ phosphorylation in C2C12 myotubes. Food Chem Toxicol. 2011; 49:2609–2617.
Article37. Kim MS, Hur HJ, Kwon DY, Hwang JT. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol Cell Endocrinol. 2012; 358:127–134.
Article38. Gannon MC, Nuttall FQ. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr Metab (Lond). 2006; 3:16.
Article39. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000; 85:2970–2973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of regular physical exercise on glucose uptake in soleus and intravenous glucose tolerance in streptozotocin diabetic rats
- Effects of insulin and exercise on glucose uptake of skeletal muscle in diabetic rats
- The Role of the Central Parasympathetic Nervous System in Modulating Glucose Metabolism in Streptozotocin-induced Diabetic Rats
- Soybean isoflavone extract improves glucose tolerance and raises the survival rate in streptozotocin-induced diabetic rats
- Hypothalamic AMPK Activity in Diabetic Rats