Diabetes Metab J.
2011 Jun;35(3):248-254. 10.4093/dmj.2011.35.3.248.
Relationship between Chemerin Levels and Cardiometabolic Parameters and Degree of Coronary Stenosis in Korean Patients with Coronary Artery Disease
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. kgpark@dsmc.or.kr
- KMID: 2175429
- DOI: http://doi.org/10.4093/dmj.2011.35.3.248
Abstract
- BACKGROUND
Chemerin is a novel adipokine that is associated with inflammation and adipogenesis. However, it remains unclear whether chemerin is involved in patients with cardiovascular disease. We investigated whether the serum chemerin levels of Korean patients with coronary artery disease correlated with specific cardiometabolic parameters.
METHODS
In total, 131 patients, all of whom had coronary artery stenosis exceeding 50%, participated in this study. Their serum chemerin levels and cardiometabolic parameters were measured. The serum chemerin levels of two groups of patients were compared; those with one stenotic vessel (n=68) and those with multiple stenotic vessels, including left main coronary artery disease (n=63).
RESULTS
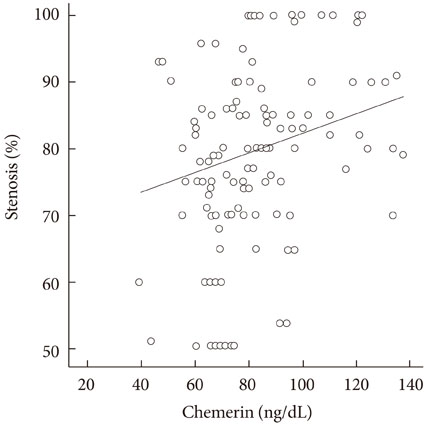
Serum chemerin levels correlated positively with the degree of coronary artery stenosis and fasting glucose, triglyceride, total cholesterol, low density lipoprotein cholesterol, and high sensitive C-reactive protein levels. The group with multiple stenotic vessels, including left main disease, had higher chemerin levels than the group with one stenotic vessel (t=-2.129, P=0.035). Multiple binary logistic regression showed chemerin was not an independent risk factor of multiple vessel disease (odds ratio, 1.018; confidence interval, 0.997 to 1.040; P=0.091).
CONCLUSION
Serum chemerin levels have a significant correlation with several cardiometabolic risk factors and the degree of coronary artery stenosis in Korean patients with coronary artery disease. However, multiple binary logistic regression showed chemerin was not an independent risk factor of multiple vessel disease. Additional investigations are necessary to fully elucidate the role of chemerin in cardiovascular disease.
MeSH Terms
-
Adipogenesis
Adipokines
C-Reactive Protein
Cardiovascular Diseases
Cholesterol
Cholesterol, LDL
Coronary Artery Disease
Coronary Stenosis
Coronary Vessels
Fasting
Glucose
Glycosaminoglycans
Humans
Inflammation
Lipoproteins
Logistic Models
Risk Factors
Adipokines
C-Reactive Protein
Cholesterol
Cholesterol, LDL
Glucose
Glycosaminoglycans
Lipoproteins
Figure
Cited by 1 articles
-
Serum Chemerin Levels are Associated with Visceral Adiposity, Independent of Waist Circumference, in Newly Diagnosed Type 2 Diabetic Subjects
Dae Young Cheon, Jun Goo Kang, Seong Jin Lee, Sung Hee Ihm, Eun Jig Lee, Moon Gi Choi, Hyung Joon Yoo, Chul Sik Kim
Yonsei Med J. 2017;58(2):319-325. doi: 10.3349/ymj.2017.58.2.319.
Reference
-
1. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983. 67:968–977.2. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006. 55:1537–1545.3. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004. 92:347–355.4. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006. 17:4–12.5. Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008. 34:2–11.6. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000. 11:327–332.7. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000. 106:473–481.8. Steffens S, Mach F. Adiponectin and adaptive immunity: linking the bridge from obesity to atherogenesis. Circ Res. 2008. 102:140–142.9. Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Kataoka T, Kamimori K, Shimodozono S, Kobayashi Y, Yoshiyama M, Takeuchi K, Yoshikawa J. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004. 90:528–533.10. Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001. 88:954–960.11. Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009. 206:239–244.12. Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005. 13:1764–1771.13. Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005. 288:H2031–H2041.14. Kowalska I. Role of adipose tissue in the development of vascular complications in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2007. 78:Suppl 1. S14–S22.15. Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007. 282:28175–28188.16. Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003. 198:977–985.17. Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005. 280:34661–34666.18. Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol. 2005. 174:244–251.19. Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007. 148:4687–4694.20. Mark DB, Nelson CI, Califf RM, Harrell FE Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, DeLong ER, Smith PD, Reves JG, Jollis JG, Tcheng JE, Muhlbaier LH, Lowe JE, Phillips HR, Pryor DB. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994. 89:2015–2025.21. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008. 359:2324–2336.22. Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Goke B, Leber AW, Parhofer KG, Broedl UC. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009. 161:339–344.23. Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009. 58:1971–1977.24. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003. 111:1805–1812.25. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003. 107:363–369.26. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000. 342:836–843.27. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000. 102:2165–2168.28. Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J Exp Med. 2008. 205:2187–2190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous Transluminal Coronary Angioplasty for Coronary Artery Stenosis in an Adult Kawasaki Disease with Coronary Aneurysm : A Case Report and Review
- Proximal Coronary Artery Stenosis after Direct Coronary Artery Ostial Perfusion : Report of 3 Cases
- A Case of Single Coronary Artery c Effort Angina
- Isolated Coronary Ostial Stenosis Confirmed by Transesophageal Echocardiogram: A Case Report
- Percutaneous transluminal coronary angioplasty for ostial stenosis of the left coronary artery