Cancer Res Treat.
2013 Mar;45(1):40-47.
Feasibility of Oxaliplatin, Leucovorin, and 5-Fluorouracil (FOLFOX-4) Chemotherapy in Heavily Pretreated Patients with Recurrent Epithelial Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. pnhkhr@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Major in Biomodulation, World Class University, Seoul National University, Seoul, Korea.
Abstract
- PURPOSE
The purpose of this study is to evaluate the efficacy and toxicity of oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX-4) chemotherapy in heavily pretreated patients with recurrent epithelial ovarian cancer (EOC).
MATERIALS AND METHODS
Clinical data were reviewed in 28 patients who received FOLFOX-4 as more than the second-line chemotherapy, consisting of 85 mg/m2 of oxaliplatin as a 2-hour infusion, 200 mg/m2 of leucovorin as a 2-hour infusion, and bolus 400 mg/m2 on day 1, followed by a 22-hour infusion of 600 mg/m2 of 5-fluorouracil for two consecutive days every three weeks. In addition, its efficacy and toxicity were compared with those reported in in three previous relevant studies.
RESULTS
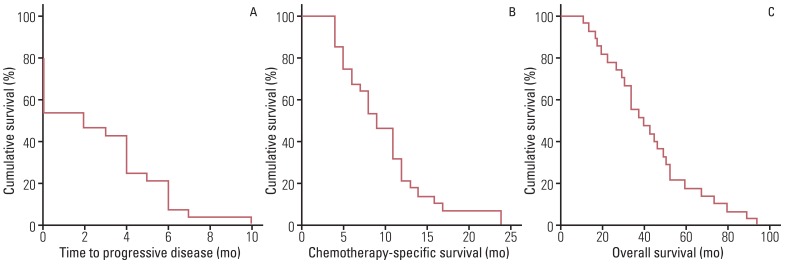
A total of 128 cycles of FOLFOX-4 were administered with the median number of five cycles (range, 1 to 10 cycles). In nine patients with measurable disease, complete response (CR) and partial response (PR) were observed in 0 (0%) and two (22.2%) patients, whereas in 19 patients with non-measurable disease, CR and PR were observed in 0 (0%) and five (26.3%) patients. Among all patients, grade 3 anemia, neutropenia, and thrombocytopenia were observed in two (7.1%), three (10.7%), and one (3.6%) patient, and grade 3 fatigue, nausea and vomiting, and peripheral neuropathy were observed in one (3.6%), two (7.1%), and three (10.7%) patients. In addition, median values of time to progressive disease and chemotherapy-specific survival were three months (range, 0 to 10 months) and nine months (range, 4 to 24 months).
CONCLUSION
FOLFOX-4 is feasible as salvage chemotherapy with acceptable toxicity for heavily pretreated patients with recurrent EOC.
Keyword
MeSH Terms
Figure
Reference
-
1. Suh DH, Kim K, Kim JW. Major clinical research advances in gynecologic cancer in 2011. J Gynecol Oncol. 2012; 23:53–64. PMID: 22355468.
Article2. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–3200. PMID: 12860964.
Article3. Markman M. Optimal management of recurrent ovarian cancer. Int J Gynecol Cancer. 2009; 19(Suppl 2):S40–S43. PMID: 19955913.
Article4. Kim YH, Kim SC. Recent advances in the biomarkers for epithelial ovarian cancer. J Gynecol Oncol. 2011; 22:219–221. PMID: 22247797.
Article5. Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998; 9:1053–1071. PMID: 9834817.
Article6. Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996; 52:1855–1865. PMID: 8951344.
Article7. Prefontaine M, Donovan JT, Powell JL, Buley L. Treatment of refractory ovarian cancer with 5-fluorouracil and leucovorin. Gynecol Oncol. 1996; 61:249–252. PMID: 8626142.8. Look KY, Muss HB, Blessing JA, Morris M. A phase II trial of 5-fluorouracil and high-dose leucovorin in recurrent epithelial ovarian carcinoma. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1995; 18:19–22. PMID: 7847253.9. Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs. 1997; 8:876–885. PMID: 9402315.
Article10. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004; 22:23–30. PMID: 14665611.
Article11. Pectasides D, Pectasides M, Farmakis D, Bountouroglou N, Nikolaou M, Koumpou M, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: a phase II study. Ann Oncol. 2003; 14:537–542. PMID: 12649097.
Article12. Sundar S, Symonds RP, Decatris MP, Kumar DM, Osman A, Vasanthan S, et al. Phase II trial of oxaliplatin and 5-fluorouracil/leucovorin combination in epithelial ovarian carcinoma relapsing within 2 years of platinum-based therapy. Gynecol Oncol. 2004; 94:502–508. PMID: 15297195.
Article13. Pectasides D, Pectasides M, Farmakis D, Gaglia A, Koumarianou A, Nikolaou M, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil (FOLFOX 4) in platinum-resistant and taxane-pretreated ovarian cancer: a phase II study. Gynecol Oncol. 2004; 95:165–172. PMID: 15385127.
Article14. Rosa DD, Awada A, Mano MS, Selleslags J, Lebrun F, Gil T, et al. Oxaliplatin/5fluorouracil-based chemotherapy was active and well tolerated in heavily pretreated patients with ovarian carcinoma. Arch Gynecol Obstet. 2008; 278:457–462. PMID: 18273626.
Article15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
Article16. Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996; 14:1545–1551. PMID: 8622070.
Article17. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0 [Internet]. 2003. cited 2012 Nov 1. Bethesda: National Cancer Institute;Available from: http://ctep.cancer.gov/forms/CTCAEv3.pdf .18. Mathe G, Kidani Y, Segiguchi M, Eriguchi M, Fredj G, Peytavin G, et al. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother. 1989; 43:237–250. PMID: 2675999.19. Dieras V, Bougnoux P, Petit T, Chollet P, Beuzeboc P, Borel C, et al. Multicentre phase II study of oxaliplatin as a single-agent in cisplatin/carboplatin +/- taxane-pretreated ovarian cancer patients. Ann Oncol. 2002; 13:258–266. PMID: 11886003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- Interstitial Lung Disease Associated with Combination Chemotherapy of Oxaliplatin, 5-Fluorouracil, and Leucovorin
- Diffuse alveolar damage during chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin
- Oxaliplatin-induced Pulmonary Fibrosis: Two Case Reports
- Oxaliplatin, 5-fluorouracil and Leucovorin (FOLFOX-4) Combination Chemotherapy as a Salvage Treatment in Advanced Gastric Cancer