J Clin Neurol.
2010 Mar;6(1):10-18. 10.3988/jcn.2010.6.1.10.
Will Molecular Optical Imaging Have Clinically Important Roles in Stroke Management, and How?
- Affiliations
-
- 1Molecular Imaging and Neurovascular Research Laboratory, Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea. kdongeog@duih.org
- 2Center for Molecular Imaging Research, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts, USA.
- 3Department of Radiology, University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
- KMID: 2135476
- DOI: http://doi.org/10.3988/jcn.2010.6.1.10
Abstract
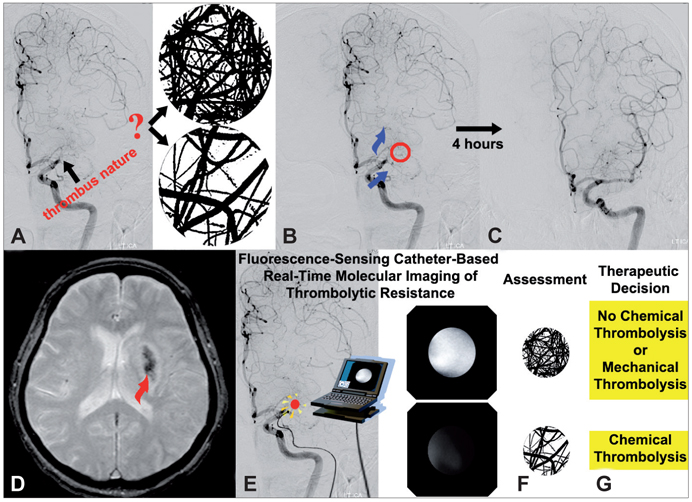
- Molecular imaging is a novel technology to visualize biological processes at the cellular and molecular levels, which is reshaping both biomedical research and clinical practice. By providing molecular information to supplement and augment conventional anatomy-based imaging, molecular imaging is expected to allow 1) the earlier detection of diseases, 2) precise evaluation of disease stages, and 3) both diagnostic and therapeutic monitoring of disease progression in a quantitative manner. In this brief review, we present our view on the prospects of molecular optical imaging in the field of stroke practice, focusing on the imaging vulnerability of atherosclerotic plaques, thrombolytic resistance, real-time cerebral perfusion, and penumbra.
Keyword
MeSH Terms
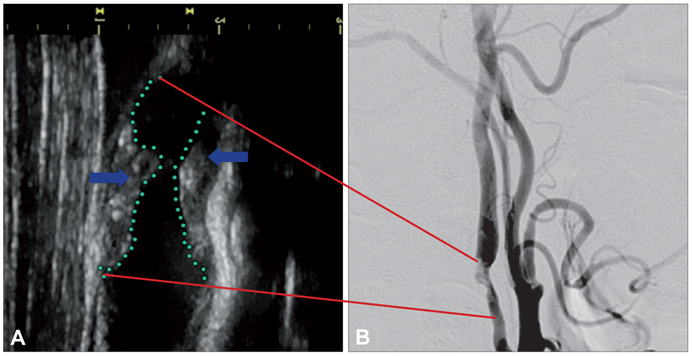
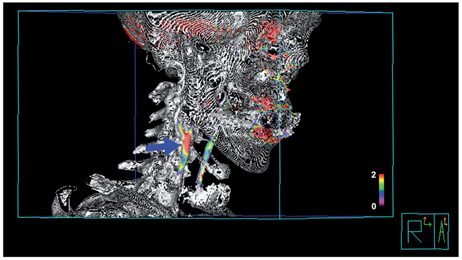
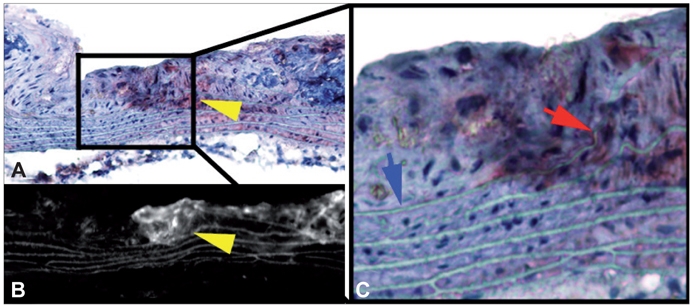
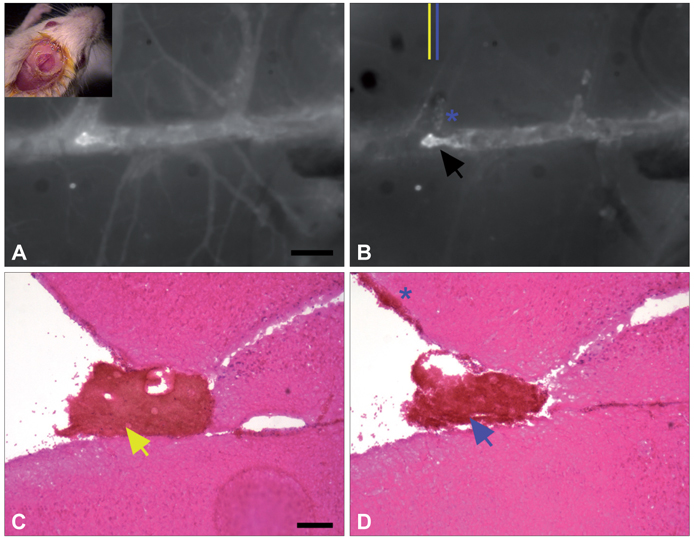
Figure
Cited by 1 articles
-
Complementarity between 18F-FDG PET/CT and Ultrasonography or Angiography in Carotid Plaque Characterization
Sang-Mi Noh, Won Jun Choi, Byeong-Teck Kang, Sang-Wuk Jeong, Dong Kun Lee, Dawid Schellingerhout, Jeong-Seok Yeo, Dong-Eog Kim
J Clin Neurol. 2013;9(3):176-185. doi: 10.3988/jcn.2013.9.3.176.
Reference
-
1. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009. 119:480–486.2. Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006. 37:1923–1932.3. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.
Article4. Lusis AJ. Atherosclerosis. Nature. 2000. 407:233–241.
Article5. Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004. 13:125–138.6. Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient-Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006. 98(2A):2H–15H.
Article7. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000. 20:1262–1275.8. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994. 343:311–322.9. Bang OY. Multimodal MRI for ischemic stroke: from acute therapy to preventive strategies. J Clin Neurol. 2009. 5:107–119.
Article10. Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000. 343:710–722.
Article11. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.12. The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997. 28:2109–2118.13. Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005. 36:2311–2320.
Article14. Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004. 35:11 Suppl 1. 2726–2730.
Article15. Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006. 12:1278–1285.
Article16. Rosell A, Foerch C, Murata Y, Lo EH. Mechanisms and markers for hemorrhagic transformation after stroke. Acta Neurochir Suppl. 2008. 105:173–178.
Article17. Diedler J, Sykora M, Blatow M, Jüttler E, Unterberg A, Hacke W. Decompressive surgery for severe brain edema. J Intensive Care Med. 2009. 24:168–178.
Article18. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009. 8:326–333.
Article19. Köhrmann M, Schwab S. Hemicraniectomy for malignant middle cerebral artery infarction. Curr Opin Crit Care. 2009. 15:125–130.
Article20. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.
Article21. Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005. 293:855–862.
Article22. Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003. 17:545–580.
Article23. Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003. 107:1092–1095.
Article24. Fayad ZA. Cardiovascular molecular imaging. Arterioscler Thromb Vasc Biol. 2009. 29:981–982.
Article25. Tempany CM, McNeil BJ. Advances in biomedical imaging. JAMA. 2001. 285:562–567.
Article26. Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001. 219:316–333.
Article27. Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008. 451:953–957.
Article28. Wong FC, Kim EE. A review of molecular imaging studies reaching the clinical stage. Eur J Radiol. 2009. 70:205–211.
Article29. Yang M, Jiang P, Hoffman RM. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2007. 67:5195–5200.
Article30. Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009. 29:983–991.
Article31. Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009. 29:1017–1024.32. Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009. 29:1009–1016.
Article33. Schwille P, Haupts U, Maiti S, Webb WW. Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys J. 1999. 77:2251–2265.
Article34. Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999. 17:375–378.
Article35. Terai T, Nagano T. Fluorescent probes for bioimaging applications. Curr Opin Chem Biol. 2008. 12:515–521.
Article36. Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005. 23:313–320.
Article37. Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003. 9:123–128.
Article38. Chang K, Jaffer F. Advances in fluorescence imaging of the cardiovascular system. J Nucl Cardiol. 2008. 15:417–428.
Article39. Funovics MA, Weissleder R, Mahmood U. Catheter-based in vivo imaging of enzyme activity and gene expression: feasibility study in mice. Radiology. 2004. 231:659–666.
Article40. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003. 2:43–53.
Article41. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998. 339:1415–1425.
Article42. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997. 336:1276–1282.
Article43. Crouse JR 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995. 92:1141–1147.
Article44. Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002. 15:439–446.
Article45. U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol. 2009. 8:569–580.
Article46. Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005. 36:2764–2772.47. Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging. 2002. 15:62–67.
Article48. Lindner JR, Song J, Xu F, Klibanov AL, Singbartl K, Ley K, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000. 102:2745–2750.
Article49. Weinberger J, Azhar S, Danisi F, Hayes R, Goldman M. A new noninvasive technique for imaging atherosclerotic plaque in the aortic arch of stroke patients by transcutaneous real-time B-mode ultrasonography: an initial report. Stroke. 1998. 29:673–676.
Article50. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. N Engl J Med. 2000. 342:1693–1700.
Article51. Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009. 84:362–387. quiz 367-368.
Article52. Usman AA, Tang GL, Eskandari MK. Metaanalysis of procedural stroke and death among octogenarians: carotid stenting versus carotid endarterectomy. J Am Coll Surg. 2009. 208:1124–1131.
Article53. Rerkasem K, Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. Eur J Vasc Endovasc Surg. 2009. 37:504–511.
Article54. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006. 47:8 Suppl. C13–C18.
Article55. Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, et al. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006. 26:1837–1844.
Article56. Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed Engl. 2008. 47:2804–2807.
Article57. Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007. 116:1052–1061.
Article58. Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, et al. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006. 114:55–62.59. Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002. 105:2766–2771.
Article60. Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007. 115:377–386.
Article61. Kim DE, Kim JY, Schellingerhout D, Shon SM, Jeong SW, Kim EJ, et al. Molecular imaging of cathepsin B proteolytic enzyme activity reflects the inflammatory component of atherosclerotic pathology and can quantitatively demonstrate the antiatherosclerotic therapeutic effects of atorvastatin and glucosamine. Mol Imaging. 2009. 8:291–301.
Article62. Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007. 115:2292–2298.
Article63. Sarai M, Hartung D, Petrov A, Zhou J, Narula N, Hofstra L, et al. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging. J Am Coll Cardiol. 2007. 50:2305–2312.
Article64. Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004. 350:1472–1473.
Article65. Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003. 108:2270–2274.
Article66. Trivedi RA, Mallawarachi C, U-King-Im JM, Graves MJ, Horsley J, Goddard MJ, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006. 26:1601–1606.
Article67. Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008. 117:379–387.
Article68. Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007. 13:636–641.
Article69. Kim DE, Tsuji K, Kim YR, Mueller FJ, Eom HS, Snyder EY, et al. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006. 241:822–830.
Article70. Kim DE, Schellingerhout D, Jaffer FA, Weissleder R, Tung CH. Near-infrared fluorescent imaging of cerebral thrombi and blood-brain barrier disruption in a mouse model of cerebral venous sinus thrombosis. J Cereb Blood Flow Metab. 2005. 25:226–233.
Article71. Jaffer FA, Tung CH, Wykrzykowska JJ, Ho NH, Houng AK, Reed GL, et al. Molecular imaging of factor XIIIa activity in thrombosis using a novel, near-infrared fluorescent contrast agent that covalently links to thrombi. Circulation. 2004. 110:170–176.
Article72. Muszbek L, Yee VC, Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999. 94:271–305.73. Robinson BR, Houng AK, Reed GL. Catalytic life of activated factor XIII in thrombi. Implications for fibrinolytic resistance and thrombus aging. Circulation. 2000. 102:1151–1157.74. Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999. 99:3050–3055.
Article75. Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem. 2004. 11:2245–2260.
Article76. Woitzik J, Peña-Tapia PG, Schneider UC, Vajkoczy P, Thomé C. Cortical perfusion measurement by indocyanine-green videoangiography in patients undergoing hemicraniectomy for malignant stroke. Stroke. 2006. 37:1549–1551.
Article77. Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke. 2009. 40:2233–2235.
Article78. Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996. 80:263–266.
Article79. Czabanka M, Peña-Tapia P, Schubert GA, Woitzik J, Vajkoczy P, Schmiedek P. Characterization of cortical microvascularization in adult moyamoya disease. Stroke. 2008. 39:1703–1709.
Article80. Pestalozza IF, Di Legge S, Calabresi M, Lenzi GL. Ischaemic penumbra: highlights. Clin Exp Hypertens. 2002. 24:517–529.
Article81. Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008. 14:497–500.
Article82. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999. 22:391–397.
Article83. Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009. 15:104–109.
Article84. Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003. 63:8122–8125.85. Choi Y, Weissleder R, Tung CH. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer Res. 2006. 66:7225–7229.
Article86. Kim DE, Kim JY, Schellingerhout D, Kim EJ, Kim HK, Lee S, et al. Protease Imaging of Human Atheromata Captures Molecular Information of Atherosclerosis, Complementing Anatomic Imaging. Arterioscler Thromb Vasc Biol. 2010. 30:449–456.
Article87. Kim DE, Jeong SW. Molecular Imaging of Atherosclerosis. J Korean Med Assoc. 2009. 52:143–150.
Article88. Kim DE, Kim JY, Kim EJ, Jeong SW. Molecular optical imaging of cathepsin-B proteolytic enzyme activity to reflect atherosclerosis pathophysiology and anti-atherosclerotic therapeutic effect. J Korean Neurol Assoc. 2009. 27:36–41.