J Korean Med Sci.
2005 Oct;20(5):888-891. 10.3346/jkms.2005.20.5.888.
Detection of Bartonella henselae DNA by Polymerase Chain Reaction in a Patient with Cat Scratch Disease: A Case Report
- Affiliations
-
- 1Department of Pediatrics, Sanggyepaik Hospital, Inje University College of Medicine, Seoul, Korea. pedchung@sanggyepaik.ac.kr
- 2Department of Diagnostic Laboratory Medicine, Sanggyepaik Hospital, Inje University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Sanggyepaik Hospital, Inje University College of Medicine, Seoul, Korea.
- 4Department of Otolaryngology, Sanggyepaik Hospital, Inje University College of Medicine, Seoul, Korea.
- 5Department of Diagnostic Pathology, Sanggyepaik Hospital, Inje University College of Medicine, Seoul, Korea.
- KMID: 1781778
- DOI: http://doi.org/10.3346/jkms.2005.20.5.888
Abstract
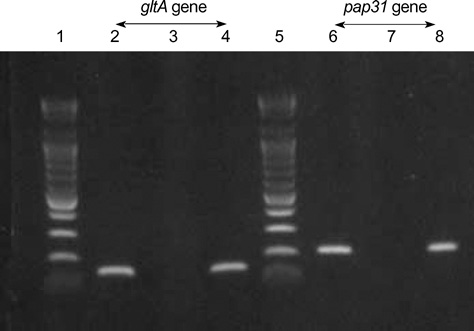
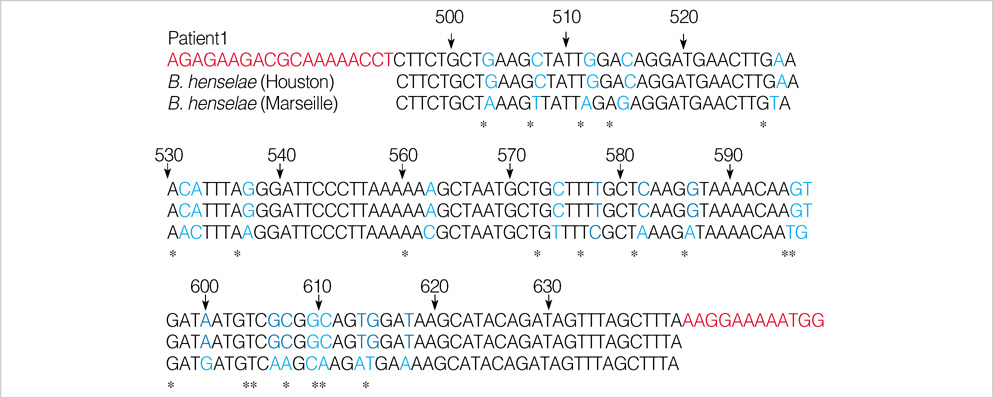
- We report a case of cat scratch disease caused by Bartonella henselae in Korea. A 25-yr-old woman developed left cervical lymphadenopathy with history of contact with a dog. The cervical lymphadenopathy persisted for 1 month and resolved gradually and spontaneously. Serologic test was not done during the acute stage of the disease. Immunofluorescent antibody test performed during the convalescent stage was positive for B. henselae. To confirm B. henselae infection, polymerase chain reaction (PCR) analysis using aspirates of cervical lymph node was performed and the presence of B. henselae DNA was demonstrated. This is the first reported case of cat scratch disease in Korea confirmed by PCR for B. henselae DNA.
MeSH Terms
Figure
Cited by 3 articles
-
Diagnosis and Treatment of Cervical Lymphadenitis from a Standpoint of Infectious Disease Specialists
Joon Young Song, Hee Jin Cheong
Infect Chemother. 2008;40(2):76-82. doi: 10.3947/ic.2008.40.2.76.Cat-Scratch Disease: A Case Report and Literature Review of Human and Animal Studies Performed in Korea
Min Hee Kim, Baek-Nam Kim, Tae Hee Han
Infect Chemother. 2012;44(4):299-302. doi: 10.3947/ic.2012.44.4.299.Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats and dogs in Korea
You-seok Kim, Kyoung-won Seo, Jong-hwa Lee, Eun-wha Choi, Hee-woo Lee, Cheol-yong Hwang, Nam-shik Shin, Hee-jeong Youn, Hwa young Youn
J Vet Sci. 2009;10(1):85-87. doi: 10.4142/jvs.2009.10.1.85.
Reference
-
1. Anderson BE, Neuman MA. Bartonella spp. As emerging human pathogens. Clin Microbiol Rev. 1997. 10:203–219.
Article2. Carithers HA. Cat-scratch disease: an overview based on a study of 1,200 patients. Am J Dis Child. 1985. 139:1124–1133.3. Regnery RL, Olson JG, Perkins BA, Bibb W. Serological response to "Rochalimaea henselae" antigen in suspected cat scratch disease. Lancet. 1992. 339:1443–1445.4. Sander A, Posselt M, Bohm N, Ruess M, Altwegg M. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. J Clin Microbiol. 1999. 37:993–997.
Article5. Margolis B, Kuzu I, Hermann M, Raible MD, Hsi E, Alkan S. Rapid polymerase chian reaction-based confirmation of cat scratch disease and Bartonella henselae infection. Arch Pathol Lab Med. 2003. 127:706–710.6. Zeaiter Z, Fournier PE, Raoult D. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J Clin Microbiol. 2002. 40:1023–1030.
Article7. Windsor JJ. Cat scratch disease: epidemiology, aetiology and treatment. Br J Biomed Sci. 2001. 58:101–110.8. Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, Umeda A, Furuya T, Kawauchi S, Sasaki K. Cat scratch disease: analysis of 130 seropositive cases. J Infect Chemother. 2002. 8:349–352.
Article9. Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998. 26:80–84.
Article10. Chae MB, Lee JY, Kwak YG, Park SH, Lim HJ, Park SW, Chung MH, Kim MK, Kang JS. Prevalence of antibodies to Bartonella henselae and Bartonella quintana in Korean patients with lymphadenopathy. Korean J Infect Dis. 2002. 34:305–310.11. Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998. 352:1682.
Article12. Keret D, Giladi M, Kletter Y, Wientoub S. Cat scratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br. 1998. 80:766–767.13. Sander A, Berner R, Ruess M. Serodiagnosis of cat scratch disease: response to Bartonella henselae in children and a review of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2001. 20:392–401.
Article14. Avidor B, Varon M, Marmor S, Lifschitz-Mercer B, Kletter Y, Ephros M, Giladi M. DNA amplification for the diagnosis of cat scratch disease in small quantity clinical specimens. Am J Clin Pathol. 2001. 115:900–909.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cat Scratch Disease Confirmed by Polymerase Chain Reaction for Bartonella henselae DNA
- Cat-Scratch Disease: A Case Report and Literature Review of Human and Animal Studies Performed in Korea
- A Report of Cat Scratch Disease in Korea Confirmed by PCR Amplification of the 16S-23S rRNA Intergenic Region of Bartonella henselae
- First Case of Bartonella henselae Bacteremia in Korea
- Prevalence of antibodies to Bartonella henselae and Bartonella quintana in Korean Patients with Lymphadenopathy