Immune Netw.
2009 Dec;9(6):265-273. 10.4110/in.2009.9.6.265.
Recombinant DNA and Protein Vaccines for Foot-and-mouth Disease Induce Humoral and Cellular Immune Responses in Mice
- Affiliations
-
- 1Department of Genetic Engineering, Faculty of Life Sciences and Technology, Sungkyunkwan University, Suwon 440-746, Korea. jsyang@skku.edu
- 2National Academy of Agricultural Science, Suwon 441-707, Korea. jsyang@skku.edu
- 3National Institute of Animal Science, Suwon 441-706, Korea.
- 4National Veterinary Research & Quarantine Service, Anyang 430-824, Korea.
- KMID: 2150655
- DOI: http://doi.org/10.4110/in.2009.9.6.265
Abstract
- BACKGROUND
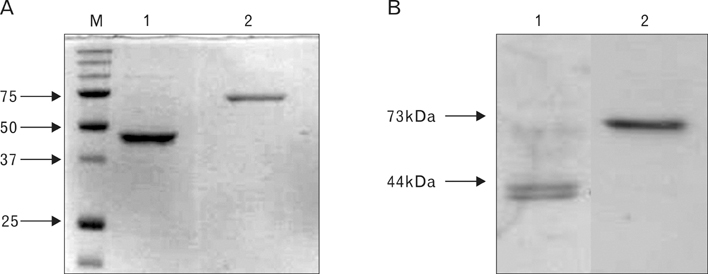
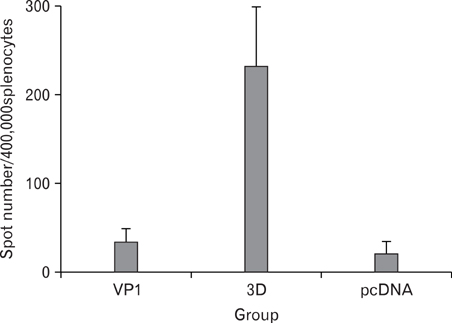
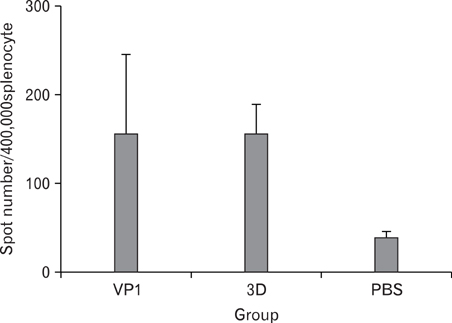
Foot-and-mouth disease virus (FMDV) is a small single-stranded RNA virus which belongs to the family Picornaviridae, genus Apthovirus. It is a principal cause of FMD which is highly contagious in livestock. In a wild type virus infection, infected animals usually elicit antibodies against structural and non-structural protein of FMDV. A structural protein, VP1, is involved in neutralization of virus particle, and has both B and T cell epitopes. A RNA-dependent RNA polymerase, 3D, is highly conserved among other serotypes and strongly immunogenic, therefore, we selected VP1 and 3D as vaccine targets. METHODS: VP1 and 3D genes were codon-optimized to enhance protein expression level and cloned into mammalian expression vector. To produce recombinant protein, VP1 and 3D genes were also cloned into pET vector. The VP1 and 3D DNA or proteins were co-immunized into 5 weeks old BALB/C mice. RESULTS: Antigen-specific serum antibody (Ab) responses were detected by Ab ELISA. Cellular immune response against VP1 and 3D was confirmed by ELISpot assay. CONCLUSION: The results showed that all DNA- and protein-immunized groups induced cellular immune responses, suggesting that both DNA and recombinant protein vaccine administration efficiently induced Ag-specific humoral and cellular immune responses.
MeSH Terms
-
Animals
Antibodies
Clone Cells
DNA
DNA, Recombinant
Enzyme-Linked Immunosorbent Assay
Enzyme-Linked Immunospot Assay
Epitopes, T-Lymphocyte
Foot-and-Mouth Disease
Foot-and-Mouth Disease Virus
Humans
Immunity, Cellular
Livestock
Mice
Picornaviridae
Proteins
RNA Replicase
RNA Viruses
Vaccines
Virion
Viruses
Antibodies
DNA
DNA, Recombinant
Epitopes, T-Lymphocyte
Proteins
RNA Replicase
Vaccines
Figure
Cited by 1 articles
-
A review of vaccine development and research for industry animals in Korea
Nak-Hyung Lee, Jung-Ah Lee, Seung-Yong Park, Chang-Seon Song, In-Soo Choi, Joong-Bok Lee
Clin Exp Vaccine Res. 2012;1(1):18-34. doi: 10.7774/cevr.2012.1.1.18.
Reference
-
1. Balamurugan V, Kumar RM, Suryanarayana VV. Past and present vaccine development strategies for the control of foot-and-mouth disease. Acta Virol. 2004. 48:201–214.2. Knowles NJ, Samuel AR, Davies PR, Midgley RJ, Valarcher JF. Pandemic strain of foot-and-mouth disease virus serotype O. Emerg Infect Dis. 2005. 11:1887–1893.
Article3. Patil PK, Suryanarayana V, Bist P, Bayry J, Natarajan C. Integrity of GH-loop of foot-and-mouth disease virus during virus inactivation: detection by epitope specific antibodies. Vaccine. 2002. 20:1163–1168.
Article4. Collen T, Dimarchi R, Doel TR. A T cell epitope in VP1 of foot-and-mouth disease virus is immunodominant for vaccinated cattle. J Immunol. 1991. 146:749–755.5. Wang JH, Liang CM, Peng JM, Shieh JJ, Jong MH, Lin YL, Sieber M, Liang SM. Induction of immunity in swine by purified recombinant VP1 of foot-and-mouth disease virus. Vaccine. 2003. 21:3721–3729.
Article6. Sanz-Parra A, Sobrino F, Ley V. Infection with foot-and-mouth disease virus results in a rapid reduction of MHC class I surface expression. J Gen Virol. 1998. 79:433–436.
Article7. Yang NS, Wang JH, Lin KF, Wang CY, Kim SA, Yang YL, Jong MH, Kuo TY, Lai SS, Cheng RH, Chan MT, Liang SM. Comparative studies of the capsid precursor polypeptide P1 and the capsid protein VP1 cDNA vectors for DNA vaccination against foot-and-mouth disease virus. J Gene Med. 2005. 7:708–717.
Article8. Borrego B, Fernandez-Pacheco P, Ganges L, Domenech N, Fernandez-Borges N, Sobrino F, Rodríguez F. DNA vaccines expressing B and T cell epitopes can protect mice from FMDV infection in the absence of specific humoral responses. Vaccine. 2006. 24:3889–3899.
Article9. Collen T, Baron J, Childerstone A, Corteyn A, Doel TR, Flint M, Garcia-Valcarcel M, Parkhouse RM, Ryan MD. Heterotypic recognition of recombinant FMDV proteins by bovine T-cells: the polymerase (P3Dpol) as an immunodominant T-cell immunogen. Virus Res. 1998. 56:125–133.
Article10. Cedillo-Barrón L, Foster-Cuevas M, Cook A, Gutiérrez-Castañeda B, Kollnberger S, Léfevre F, Parkhouse RM. Immunogenicity of plasmids encoding T and B cell epitopes of foot-and-mouth disease virus (FMDV) in swine. Vaccine. 2003. 21:4261–4269.
Article11. Eriksson E, Yao F, Svensjo T, Winkler T, Slama J, Macklin MD, Andree C, McGregor M, Hinshaw V, Swain WF. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998. 78:85–91.12. Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci U S A. 1994. 91:9519–9523.
Article13. Bautista EM, Ferman GS, Gregg D, Brum MC, Grubman MJ, Golde WT. Constitutive expression of alpha interferon by skin dendritic cells confers resistance to infection by foot-and-mouth disease virus. J Virol. 2005. 79:4838–4847.
Article14. Yang JS, Kim JJ, Hwang D, Choo AY, Dang K, Maguire H, Kudchodkar S, Ramanathan MP, Weiner DB. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999). J Infect Dis. 2001. 184:809–816.
Article15. Kim SA, Liang CM, Cheng IC, Cheng YC, Chiao MT, Tseng CJ, Lee F, Jong MH, Tao MH, Yang NS, Liang SM. DNA vaccination against foot-and-mouth disease via electroporation: study of molecular approaches for enhancing VP1 antigenicity. J Gene Med. 2006. 8:1182–1191.
Article16. Park JH, Kim SJ, Oem JK, Lee KN, Kim YJ, Kye SJ, Park JY, Joo YS. Enhanced immune response with foot and mouth disease virus VP1 and interleukin-1 fusion genes. J Vet ScI. 2006. 7:257–262.
Article17. Cedillo-Barrón L, Foster-Cuevas M, Belsham GJ, Lefévre F, Parkhouse RM. Induction of a protective response in swine vaccinated with DNA encoding foot-and-mouth disease virus empty capsid proteins and the 3D RNA polymerase. J Gen Virol. 2001. 82:1713–1724.
Article18. Zhang Q, Zhu MW, Yang YQ, Shao M, Zhang ZY, Lan HY, Yan WY, Wu JJ, Zheng ZX. A recombinant fusion protein and DNA vaccines against foot-and-mouth disease virus type Asia 1 infection in guinea pigs. Acta Virol. 2003. 47:237–243.19. van Lierop MJ, Wagenaar JP, van Noort JM, Hensen EJ. Sequences derived from the highly antigenic VP1 region 140 to 160 of foot-and-mouth disease virus do not prime for a bovine T-cell response against intact virus. J Virol. 1995. 69:4511–4514.
Article20. Chinsangaram J, Beard C, Mason PW, Zellner MK, Ward G, Grubman MJ. Antibody response in mice inoculated with DNA expressing foot-and-mouth disease virus capsid proteins. J Virol. 1998. 72:4454–4457.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant DNA and Protein Vaccines for Foot-and-mouth Disease Induce Humoral and Cellular Immune Responses in Mice
- Construction of Recombinant DNA with F and HN Genes of Newcastle Disease Virus and Its Immunogenicity
- DNA immunization of Mycobacterium tuberculosis resuscitation-promoting factor B elicits polyfunctional CD8+ T cell responses
- DNA-mediated immunization of mice with plasmid encoding HBs antigen
- Potentiality of Anti-idiotypic Antibodies Mimicking GD2 to Induce Cellular Immunity