Korean J Ophthalmol.
2011 Feb;25(1):42-47. 10.3341/kjo.2011.25.1.42.
Gene Expression Changes in a Rat Model of Oxygen-Induced Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea. inyoung@gnu.ac.kr
- 2Institute of Health Science, Gyeongsang National University, Jinju, Korea.
- KMID: 994411
- DOI: http://doi.org/10.3341/kjo.2011.25.1.42
Abstract
- PURPOSE
To identify altered patterns of retinal mRNA expression in a rat model of oxygen-induced retinopathy (OIR).
METHODS
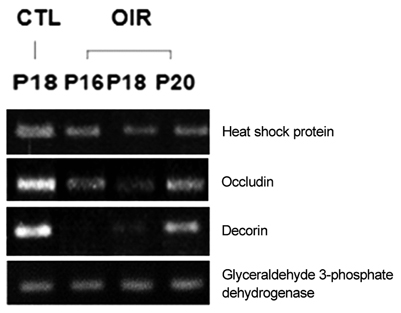
Sprague-Dawley rats from P2 to P14 were exposed to hyperoxia (80% oxygen) to induce OIR and then returned to normoxic conditions. Control rats were sustained in room air. Retinal gene expression between the rats of OIR and the controls was compared using cDNA microarray analysis. Reverse transcriptase polymerase chain reaction (RT-PCR) was used to verify the microarray results.
RESULTS
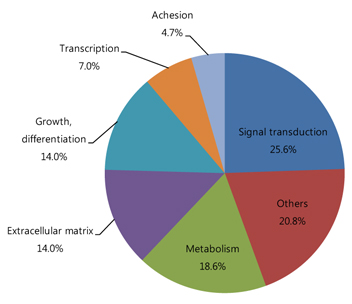
Among a total of 12,731 cDNAs analyzed by mircroarray, 13 genes were strongly up- or down-regulated (>2-fold change over controls) in the OIR rats. We found a significant increase in expression of 10 genes (CaM-kinase II inhibitor; acidic nuclear phosphoprotein 32 family, member A; vascular endothelial growth factor; interferon alpha-inducible protein 27-like; similar to enthoprotin, epsin 4, clathrin interacting protein; nidogen [entactin]; tubulin, beta5; fibrillin-1; spectrin beta2; and stearoyl-coenzyme A desaturase 2) and a significant decrease in expression of 3 genes (myelin-associated oligodendrocytic basic protein, heat shock protein, and decorin) in OIR rats compared to controls.
CONCLUSIONS
We confirmed changes in expressions of various retinal genes in a rat model of OIR by microarray and RT-PCR. This study should contribute to the understanding of genetic indicators associeated with OIR.
MeSH Terms
Figure
Reference
-
1. Sato T, Kusaka S, Hashida N, et al. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009. 93:96–103.2. van Wijngaarden P, Brereton HM, Gibbins IL, et al. Kinetics of strain-dependent differential gene expression in oxygen-induced retinopathy in the rat. Exp Eye Res. 2007. 85:508–517.3. van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Hereditary influences in oxygen-induced retinopathy in the rat. Doc Ophthalmol. 2010. 120:87–97.4. van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Stability of housekeeping gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis. 2007. 13:1508–1515.5. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003. 121:1684–1694.6. Chan-Ling T, Tout S, Holländer H, Stone J. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992. 33:2128–2147.7. Zhang W, Ito Y, Berlin E, et al. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003. 44:3119–3123.8. Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995. 15(7 Pt 1):4738–4747.9. Reynaud X, Dorey CK. Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci. 1994. 35:3169–3177.10. Min XJ, Zhou QJ, Liu T, et al. Expression of mouse telomerase reverse transcription in a mouse model of oxygen-induced retinopathy. Zhonghua Yan Ke Za Zhi. 2009. 45:199–205.11. Budd SJ, Hartnett ME. Increased angiogenic factors associated with peripheral avascular retina and intravitreous neovascularization: a model of retinopathy of prematurity. Arch Ophthalmol. 2010. 128:589–595.12. Zhang ZH, Jiang L, Qiao LX. Expression of mRNA of vascular endothelial growth factor in a rat model of hyperoxia-induced retinopathy. Zhongguo Dang Dai Er Ke Za Zhi. 2007. 9:371–374.13. Kim YH, Chung IY, Choi MY, et al. Triamcinolone suppresses retinal vascular pathology via a potent interruption of proinflammatory signal-regulated activation of VEGF during a relative hypoxia. Neurobiol Dis. 2007. 26:569–576.14. Kim YH, Kim YS, Kang SS, et al. Expression of 14-3-3 zeta and interaction with protein kinase C in the rat retina in early diabetes. Diabetologia. 2005. 48:1411–1415.15. Noh HS, Lee HP, Kim DW, et al. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res. 2004. 129:80–87.16. Simons BD, Flynn JT. Retinopathy of prematurity and associated factors. Int Ophthalmol Clin. 1999. 39:29–48.17. Madan A, Penn JS. Animal models of oxygen-induced retinopathy. Front Biosci. 2003. 8:d1030–d1043.18. Ozaki H, Seo MS, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000. 156:697–707.19. Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003. 22:1134–1146.20. Sarlos S, Rizkalla B, Moravski CJ, et al. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003. 163:879–887.21. Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009. 50:3360–3365.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Curcumin in a Mouse Model of Oxygen-Induced Retinopathy
- A Study on the Expression of Bad Gene during the Corpus Luteum Regression of Rat
- Triamcinolone Acetonide Prevents Enhancement of Hypoxia-induced Neuronal and Inducible Nitric Oxide Synthases in the Retinas of Rats with Oxygen-induced Retinopathy
- Retinal Protective Effects of Resveratrol via Modulation of Nitric Oxide Synthase on Oxygen-induced Retinopathy
- Retinal Protective Effects of Minocycline via Anti-apoptosis on Oxygen-induced Retinopathy in Neonatal Rats