Korean J Ophthalmol.
2010 Jun;24(3):155-158. 10.3341/kjo.2010.24.3.155.
The Effect of Intravitreal Bevacizumab in Patients with Acute Central Serous Chorioretinopathy
- Affiliations
-
- 1Department of Ophthalmology, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea. jiwoneye@hallym.or.kr
- KMID: 945985
- DOI: http://doi.org/10.3341/kjo.2010.24.3.155
Abstract
- PURPOSE
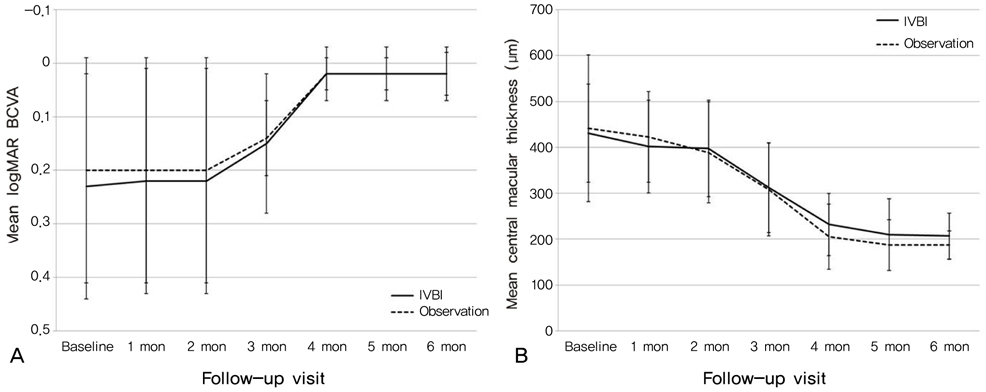
To evaluate the effect of intravitreal bevacizumab injection (IVBI) in acute central serous chorioretinopathy (CSC) patients. METHODS: Patients with acute CSC received IVBI (1.25 mg/0.05 mL) or observation by randomization. Twelve eyes in each group completed 6 months of regular follow-up and were ultimately included in this study. Each patient was assessed using best corrected visual acuity measurements, fluorescein angiography, and optical coherence tomography at baseline and had regular follow-ups after treatment. RESULTS: All patients showed improvements in visual acuity and fluorescein angiographic leakage and had resolution of their neurosensory detachment following treatment. There were no significant differences in visual acuity, central retinal thickness, or remission duration between the IVBI group and the control group at baseline or after treatment (p>0.05). CONCLUSIONS: Intravitreal bevacizumab showed no positive effect in acute CSC patients compared to the observation group, and there were no adverse effects of treatment. Further investigation will be helpful to understand this therapy in patients with CSC.
MeSH Terms
-
Acute Disease
Adult
Antibodies, Monoclonal/*administration & dosage
Capillary Permeability/drug effects
Central Serous Chorioretinopathy/*drug therapy/physiopathology
Female
Follow-Up Studies
Humans
Injections, Intraocular
Male
Middle Aged
Treatment Failure
Vascular Endothelial Growth Factor A/*antagonists & inhibitors
Visual Acuity/drug effects
Vitreous Body
Figure
Reference
-
1. Gass JD. Pathogenesis of disciform detachment of neuroepithelium: II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967. 63:587–615.2. Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989. 96:854–859.3. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002. 22:19–24.4. Chan WM, Lai TY, Lai RY, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008. 115:1756–1765.5. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008. 86:126–145.6. Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002. 133:373–385.7. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008. 246:1235–1239.8. Eandi CM, Ober M, Iranmanesh R, et al. Acute central serous chorioretinopathy and fundus autofluorescence. Retina. 2005. 25:989–993.9. Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye. 2004. 18:262–268.10. Sharma T, Shah N, Rao M, et al. Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004. 111:1708–1714.11. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363–372.12. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006. 26:383–390.13. Heiduschka P, Fietz H, Hofmeister S, et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007. 48:2814–2823.14. Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006. 26:262–269.15. Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003. 110:15–21.16. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994. 14:231–242.17. Seong HK, Bae JH, Kim ES, et al. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009. 223:343–347.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Short-term Effect of Intravitreal Bevacizumab for Treatment of Central Serous Chorioretinopathy
- Factors Influencing the Effect of the Intravitreal Bevacizumab Injection in Patients with Central Serous Chorioretinopathy
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- The Efficacy of Intravitreal Bevacizumab Injection in Patients with Acute Central Serous Chorioretinopathy
- A Case of Central Serous Chorioretinopathy Associated With Retinal Detachment Improved by Intravitreal Bevacizumab Injection