Korean J Ophthalmol.
2009 Dec;23(4):259-265. 10.3341/kjo.2009.23.4.259.
Comparison Between Intravitreal Bevacizumab and Triamcinolone for Macular Edema Secondary to Branch Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. sungpyo@hanafos.com
- KMID: 754760
- DOI: http://doi.org/10.3341/kjo.2009.23.4.259
Abstract
- PURPOSE
To compare the effects of intravitreal bevacizumab to those of triamcinolone acetonide injection for the treatment of macular edema secondary to branch retinal vein occlusion.
METHODS
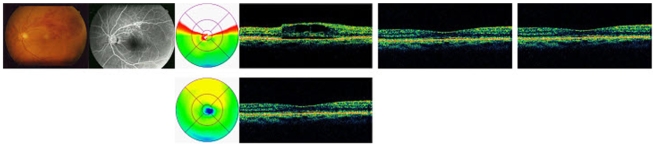
This retrospective study included 50 eyes of 50 patients who received a single injection of intravitreal bevacizumab (1.25 mg/0.05 mL, 22 eyes) or triamcinolone acetonide (4 mg/0.1 mL, 28 eyes) as the only treatment for macular edema secondary to branch retinal vein occlusion; all patients had a post-injection follow-up duration of >24 weeks. Best corrected visual acuity (BCVA), intraocular pressure (IOP), and central macular thickness (CMT) by optical coherence tomography were measured for up to 24 weeks after injection.
RESULTS
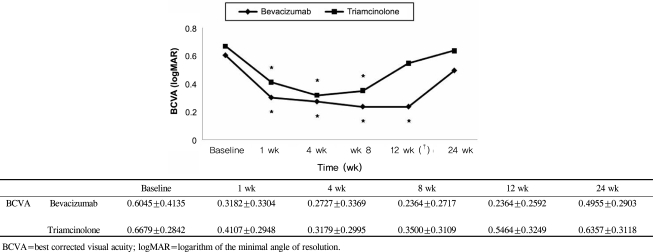
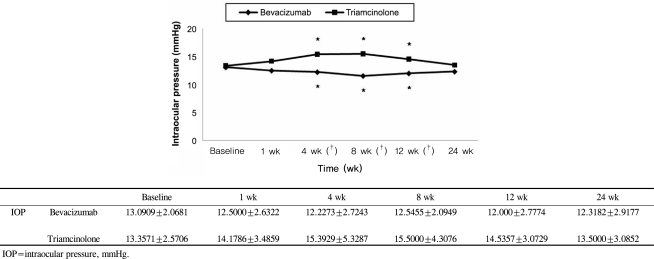
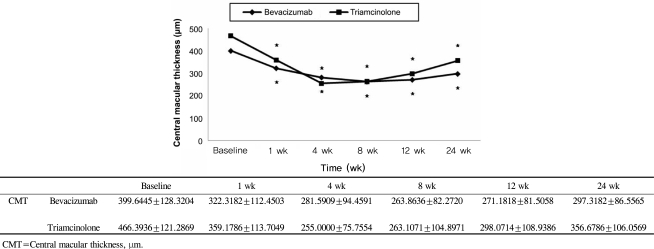
BCVA was improved at 1, 4, 8,12 weeks post-injection in the bevacizumab group, and at 1, 4, 8 weeks post-injection in the triamcinolone group. No significant difference was found between the two groups except at 12 weeks. CMT decreased significantly within each group, and no significant difference between groups was found. In the bevacizumab group, no elevated IOP was observed, whereas IOP was significantly increased at 4, 8, and 12 weeks after triamcinolone injection; IOP was therefore significantly different between the two groups.
CONCLUSIONS
Intravitreal bevacizumab is a comparatively simple treatment method that can effectively improve BCVA and reduce CMT without ocular and systemic complications. Consequently, intravitreal bevacizumab injections may be useful as both an alternative and primary treatment for macular edema secondary to branch retinal vein occlusion.
MeSH Terms
-
Adult
Aged
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal/*administration & dosage
Female
Follow-Up Studies
Glucocorticoids/*administration & dosage
Humans
Injections
Macular Edema/diagnosis/*drug therapy/etiology
Male
Middle Aged
Retinal Vein Occlusion/*complications/diagnosis
Retrospective Studies
Tomography, Optical Coherence
Treatment Outcome
Triamcinolone Acetonide/*administration & dosage
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity
Vitreous Body
Figure
Cited by 1 articles
-
Natural Short-term Course of Recurrent Macular Edema Following Intravitreal Bevacizumab Therapy in Branch Retinal Vein Occlusion
Su Jin Yoo, Jae Hui Kim, Tae Gon Lee, Jong Woo Kim, Sung Won Cho, Jung Il Han
Korean J Ophthalmol. 2017;31(2):95-101. doi: 10.3341/kjo.2017.31.2.95.
Reference
-
1. Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossing in branch retinal vein occlusion. Am J Ophthalmol. 1990; 109:298–302. PMID: 2309862.2. The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:271–282. PMID: 6383055.3. Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983; 90:458–474. PMID: 6877778.
Article4. Wallow IH, Danis RP, Bindley C, Neider M. Cystoid degeneration in experimental branch vein occlusion. Ophthalmology. 1988; 95:1371–1379. PMID: 3226685.5. Cheng KC, Wu WC. Intravitreal triamcinolone acetonide for patients with macular edema due to branch retinal vein occlusion. Kaohsiung J Med Sci. 2006; 22:321–330. PMID: 16849100.
Article6. Cakir M, Dogan M, Bayraktar Z, et al. Efficacy of intravitreal triamcinolone for the treatment of macular edema secondary to branch retinal vein occlusion in eyes with or without grid laser photocoagulation. Retina. 2008; 28:465–472. PMID: 18327140.
Article7. Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998; 341:309–315. PMID: 9543253.
Article8. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005; 25:851–855. PMID: 16205563.
Article9. Battaglia Parodi M, Saviano S, Ravalico G. Grid laser treatment in macular branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 1999; 237:1024–1027. PMID: 10654172.
Article10. Jaissle GB, Ziemssen F, Petermeier K, et al. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmology. 2006; 103:471–475.11. Iturralde D, Spide RF, Meyerle CB, et al. Intravitreal bevzcizumab (avastin) treatment of macular edema in central retinal vein occlusion: a short term study. Retina. 2006; 26:279–284. PMID: 16508427.12. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–339. PMID: 16156153.
Article13. Lang GE, Handel A. Results of laser coagulations of branch retinal vein occlusions. Klin Monatsbl Augenheilkd. 1993; 203:180–188. PMID: 8264208.14. Estrick E, Subramanian ML, Heier JS, et al. Multiple laser treatments for macular edema attributable to branch retinal vein occlusion. Am J Ophthalmol. 2005; 139:653–657. PMID: 15808160.15. Hayreh SS, Rubenstein L, Podhajsky P. Argon laser scatter photocoagulation in treatment of branch retinal vein occlusion: a prospective trial. Ophthalmologica. 1993; 206:1–14. PMID: 7506400.16. Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997; 46:1473–1480. PMID: 9287049.
Article17. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109. PMID: 9046048.18. Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphrylation of tight junction proteins occluding and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999; 274:23463–23467. PMID: 10438525.19. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal Bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–425. PMID: 17420692.
Article20. Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2008; 28:212–219. PMID: 18301025.21. Badalà F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol. 2008; 19:234–238.
Article22. Krepler K, Ergun E, Sacu S, et al. Intravitreal triamcinolone acetonide in patients with macular edema due to central retinal vein occlusion. Acta Ophthalmol Scand. 2005; 83:71–75. PMID: 15715561.23. Choi CU, Seo SW, Yang YS. Different effect of IVTA in the management of macular edema secondary to perfusion and ischemic type BRVO. J Korean Ophthalmol Soc. 2007; 48:49–54.24. Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Retina. 1981; 1:27–55. PMID: 15633406.25. Glacet-Bernard A, Coscas G, Chabanel A, et al. Prognostic factors for retinal vein occlusion: prospectivge study of 175 cases. Ophthalmology. 1996; 103:551–560. PMID: 8618752.26. Clemett RS, Kohner EM, Hamilton AM. The visual prognosis in retinal branch vein occlusion. Trans Ophthamol Soc UK. 1973; 93:523–535.27. Shilling JS, Jones CA. Retinal branch vein occlusion: a study of argon laser photocoagulation in the treatment of macular edema. Br J Ophthalmol. 1984; 68:196–198. PMID: 6365157.28. Kang SJ, Chin HS, Moon YS. Visual prognosis of macular edema associated with macular ischemia in branch retinal vein occlusion. J Korean Ophthalmol Soc. 2002; 43:1621–1628.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Triamcinolone Versus Bevacizumab for Treatment of Macular Edema Secondary to Branch Retinal Vein Occlusion
- Combined Low Dose Bevacizumab-triamcinolone versus Bevacizumab Single Intravitreal Injection for Branch Retinal Vein Occlusion
- Short-term Effectiveness of Intravitreal Triamcinolone Injection for Refractory Macular Edema Secondary to Branch Retinal Vein Occlusion
- Combined Therapy of Intravitreal Bevacizumab and Posterior Subtenon Triamcinolone Injection in Macular Edema with Branch Retinal Vein Occlusion
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema