Korean J Ophthalmol.
2007 Dec;21(4):238-243. 10.3341/kjo.2007.21.4.238.
Effects of Heme Oxygenase-1 Inducer and Inhibitor on Experimental Autoimmune Uveoretinitis
- Affiliations
-
- 1Department of Ophthalmology, Wonkwang University, Icksan, Korea. ysyang@wonkwang.ac.kr
- 2Genome Research Center for Immune Disorders, Department of Microbiology & Immunology, Wonkwang University, Icksan, Korea.
- KMID: 754643
- DOI: http://doi.org/10.3341/kjo.2007.21.4.238
Abstract
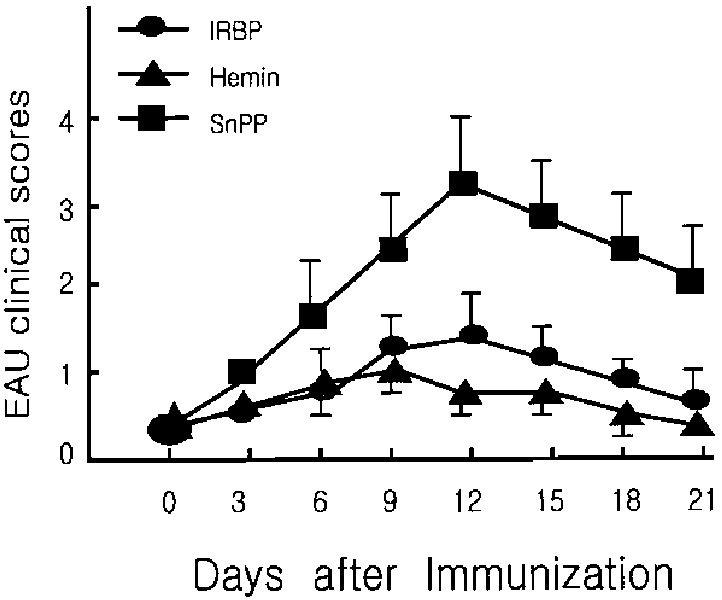
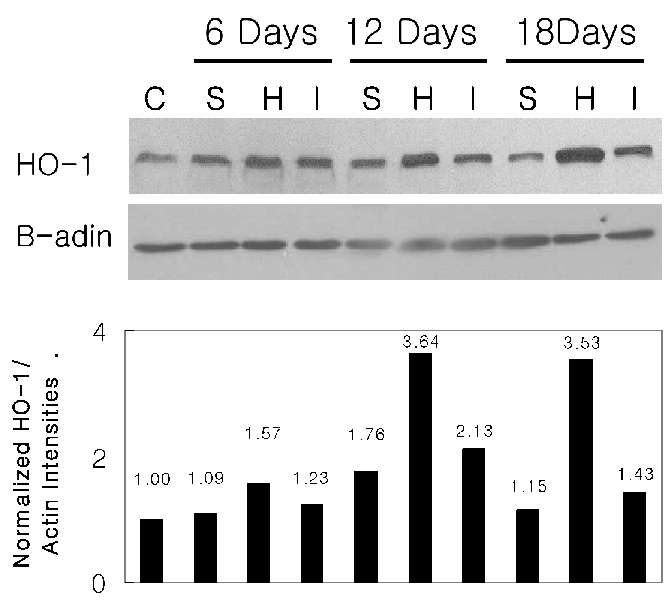
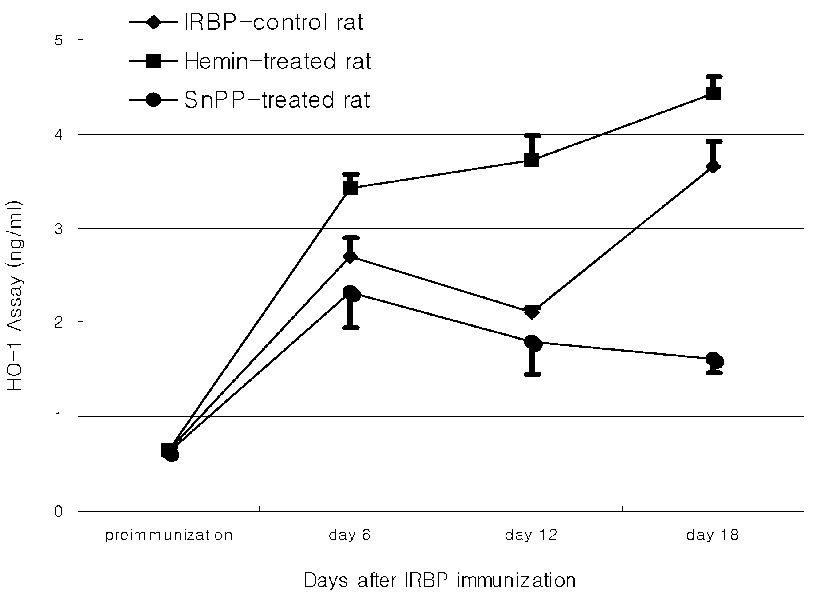
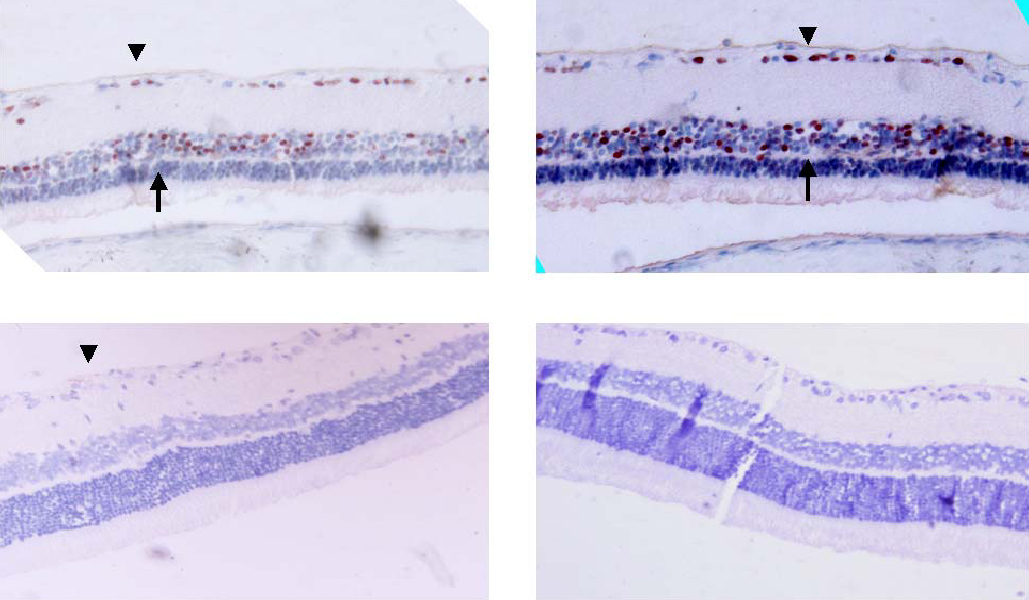
- PURPOSE: Experimental autoimmune uveoretinitis (EAU) is an animal model of posterior uveitis and heme oxygenase-1 (HO-1) is a well-known anti-oxidant factor. However, there is no report a protective role of HO-1 on EAU in vivo. To verify that HO-1 is induced in EAU by interphotoreceptor retinoid-binding protein (IRBP), that an HO-1 inducers ameliorates the associated inflammation, and that an HO-1 inhibitor exacerbates this inflammation. METHODS: Forty four Lewis rats were given either 40 mol/kg hemin or 40 mol/kg SnPP (tin protoporphyrin IX) by intraperitoneal injection and twenty two uveitis control rats were injected with 0.5 mL of saline once daily 5-20 days after IRBP immunization inducing EAU. Three normal control rats were used for Western blotting and ELISA assay of HO-1. The clinical uveitis signs of inflammation were scored in the three groups from 0 to 4 on alternate three days. To confirm the clinical results, histological and immunohistochemical stain of HO-1 were performed on the day of peak inflammation and Western blotting and ELISA assay of HO-1 were performed on 6th, 12th and 18th day after IRBP immunization. RESULTS: Hemin, an inducer of HO-1, ameliorated the clinical signs of EAU. In contrast, SnPP-treated rats show that the severity of the clinical sign were exacerbated at the peak period of the disease. These results are roughly compatible with histological, immunoblotting, and immunohistochemical evaluations and an ELISA assay of HO-1. CONCLUSIONS: We suggest that HO-1 plays an important protective role in EAU.
Keyword
MeSH Terms
-
Animals
Autoimmune Diseases/diagnosis/*drug therapy/metabolism
Blotting, Western
Disease Models, Animal
Enzyme Inhibitors/*administration & dosage
Enzyme-Linked Immunosorbent Assay
Heme Oxygenase-1/*biosynthesis/drug effects
Hemin/*administration & dosage
Immunohistochemistry
Injections, Intraperitoneal
Male
Metalloporphyrins/*administration & dosage
Microscopy, Acoustic
Protoporphyrins/*administration & dosage
Rats
Rats, Inbred Lew
Retinitis/diagnosis/*drug therapy/metabolism
Treatment Outcome
Uveitis, Posterior/diagnosis/*drug therapy/metabolism
Figure
Cited by 1 articles
-
Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
Hyun-Ock Pae, Hun-Taeg Chung
Immune Netw. 2009;9(1):12-19. doi: 10.4110/in.2009.9.1.12.
Reference
-
1. Caspi RR, Roberge FG, McAllister CG, et al. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986. 136:928–933.2. Kim HS, Lee EH, Sungjoo KY, Joo CK. Immunopathogenesis of experimental melanin protein induced uveitis. Korean J Ophthalmol. 1998. 12:14–18.3. Gery I, Mochizuki M, Nussenblatt RB. Retina specific antigens and immunopathogenic processes they provoke. Prog Retinal Res. 1986. 5:75.4. Ulyanova T, Szėl A, Kutty RK. Oxidative stress induced heme oxygenase-1 immunoreactivity in Möller cells of mouse retina in organ culture. Invest Ophthalmol Vis Sci. 2001. 42:1370–1374.5. Kutty RK, Kutty G, Wiggert B, et al. Induction of heme oxygenase-1 in the retina by intense visible light: suppression by the antioxidant dimethylthiourea. Proc Natl Acad Sci USA. 1995. 92:1177–1181.6. Ohta K, Kikuchi T, Arai S, et al. Protective role of heme oxygenase-1 against endotoxin-induced uveitis in rats. Exp Eye Res. 2003. 77:665–673.7. Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000. 60:1121–1128.8. Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Bioche. 1996. 6:129–168.9. Sardana MK, Kappas A. Dual control mechanism for heme oxygenase: tin protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc Natl Acad Sci USA. 1987. 84:2464–2468.10. Borst DE, Redmond TM, Elser JE, et al. Interphotoreceptor retinoid-binding protein. Gene characterization, protein repeat structure, and its evolution. J Biol Chem. 1989. 264:1115–1123.11. Kwak HJ, Yang YS, Pae HO, et al. Exogenous nitric oxide inhibits experimental autoimmune uveoretinitis development in Lewis rats by modulation of the Thl dependent immune response. Mol Cells. 2001. 12:178–184.12. Abraham NG, Lin JH, Dunn MW, Schwartzman ML. Presence of heme oxygenase and NADPH cytochrome P-450 (c) reductase in human corneal epithelium. Invest Ophthalmol Vis Sci. 1987. 28:1464–1472.13. Chung H, Choi DG. Clinical Analysis of Uveitis. Korean J Ophthalmol. 1989. 3:33–37.14. Siow RCM, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovase Res. 1999. 41:385–394.15. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.16. Marilena G. New physiological importance of two classic residual products: carbon monoxide and bilirubin. Biochem Mol Med. 1997. 61:136–142.17. Riedl AG, Watts PM, Brown CT, Jenner P. P450 and heme oxygenase enzymes in the basal ganglia and their roles in Parkinson's disease. Adv Neurol. 1999. 80:271–286.18. Kikuchi G, Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem. 1983. 53:163–183.19. Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987. 235:1043–1046.20. De Flora S, Rosenkranz HS, Klopman G. Structural basis of antimutagenicity of chemicals towards 4-nitroquinoline 1-oxide in Salmonella typhimurium. Mutagenesis. 1994. 9:39–45.21. Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000. 6:422–488.22. Mancuso C, Tringali G, Grossman A. The generation of nitric oxide and carbon monoxide produces opposite effects on the release of immunoreactive interleukin-1 beta from rat hypothalamus in vitro: evidence for the involvement of different signaling pathways. Endocrinology. 1998. 139:1031–1037.23. Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with oxide in the control of cellular homeostasis. Free Radic Res. 1999. 31:459–475.24. Andreoli TE. Free radicals and oxidative stress. Am J Med. 2000. 108:650–651.25. Liu Y, Zhu B, Luo L, et al. Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. Neuroreport. 2001. 12:1841–1845.26. Schluesener HJ, Seid K. Heme oxygenase-1 in lesions of rat experimental autoimmune encephalomyelitis and neuritis. J Neuroimmunol. 2000. 110:114–120.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- S Antigen Specific Rat Helper T Cell Line Induced Experimental Autoimmune Uveoretinitis
- Modulation of Melanogenesis by Heme Oxygenase-1 via p53 in Normal Human Melanocytes
- Effect of heme oxygenase induction by NO donor on the aortic contractility
- Effect of the Heme Oxygenase Inhibitor on the Hypoxic Ischemic Brain Injury in the Neonatal Rat