Korean J Ophthalmol.
2005 Sep;19(3):219-226. 10.3341/kjo.2005.19.3.219.
Neuroprotective Effect of Citicoline on Retinal Cell Damage Induced by Kainic Acid in Rats
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Gyeongsang National University, Jinju, Korea. jmpark@gshp.gsnu.ac.kr
- KMID: 754428
- DOI: http://doi.org/10.3341/kjo.2005.19.3.219
Abstract
- PURPOSE
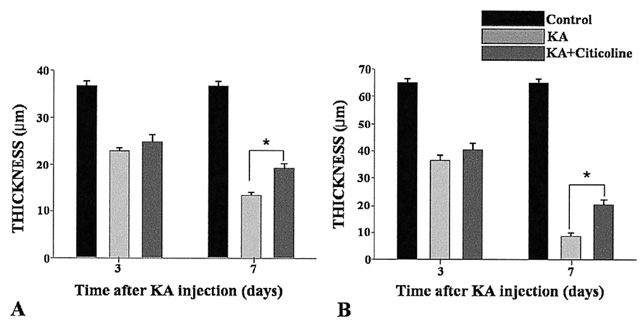
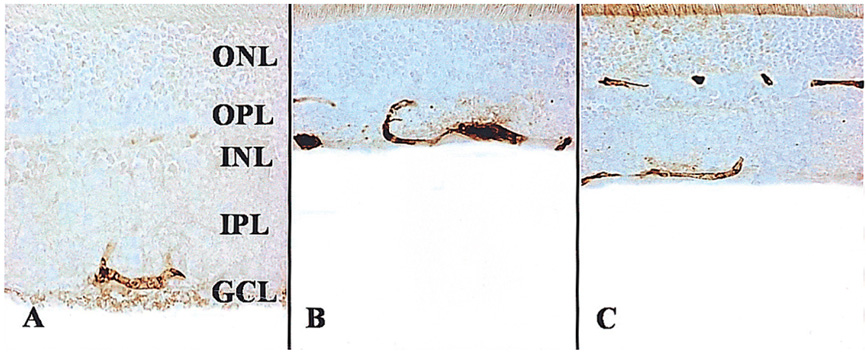
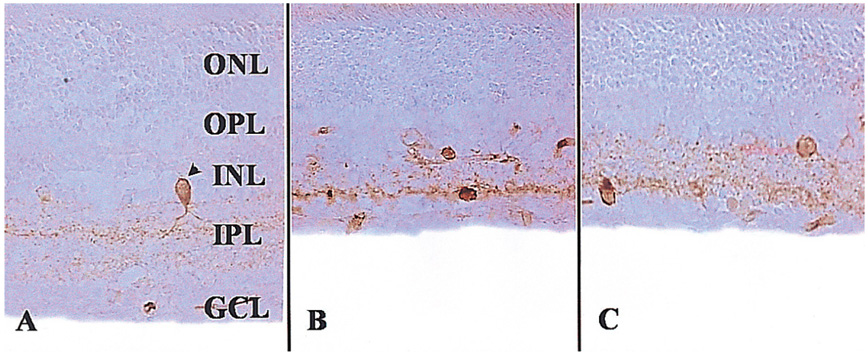
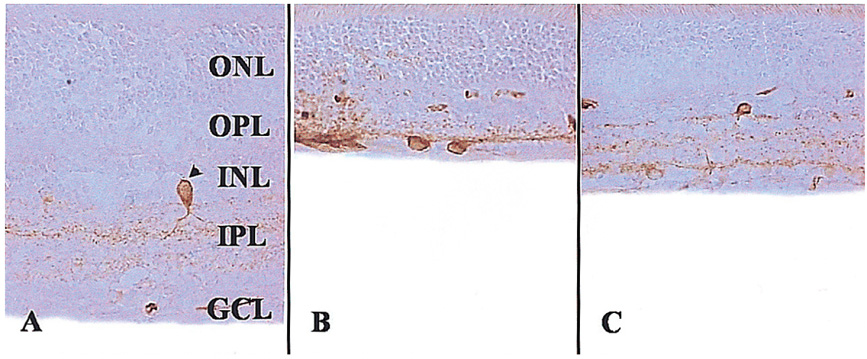
To examine whether citicoline has a neuroprotective effect on kainic acid (KA) -induced retinal damage. METHODS: KA (6 nmol) was injected into the vitreous of rat eyes. Citicoline (500mg/kg, i.p.) was administered to the rats once before and twice a day after KA-injection for 3- and 7-day intervals. The neuroprotective effects of citicoline were estimated by measuring the thickness of the various retinal layers using hematoxylin-eosin (H and E) staining. In addition, immunohistochemistry was conducted to elucidate the expression of endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS). RESULTS: Morphometric analysis of retinal damage in KA-injected eyes showed significant cell loss in the inner nuclear layer (INL) and inner plexiform layer (IPL) of the retinas at 3 and 7 days after KA injection, but not in the outer nuclear layer (ONL). At 3 days after citicoline treatment, no significant changes were detected in the retinal thickness and immunoreactivities of eNOS and nNOS. The immunoreactivities of eNOS and nNOS increased in the retina at 7 days after the KA injection. However, prolonged treatment for 7 days significantly attenuated the immunoreactivities and the reduction of thickness. CONCLUSIONS: The results indicate that citicoline has a neuroprotective effect on KA-induced neurotoxicity in the retina.
Keyword
MeSH Terms
Figure
Reference
-
1. Osborne NN, Schwarz M, Pergande G. Protection of rabbit retina from ischemic injury by flupirtine. Invest Ophthalmol Vis Sci. 1996. 37:274–280.2. Lam TT, Siew E, Chu R, Tso MO. Ameliorative effect of MK801 on retinal ischemia. J Ocular Pharmacol Ther. 1997. 13:129–137.3. Emery JM, Landis D, Paton D, et al. The lamina cribrosa in normal and glaucomatous human eyes. Trans Am Acad Ophthalmol Otolaryngol. 1974. 78:290–297.4. Quigley HA, Guy J, Anderson DR. Blockade of rapid axonal transport. Effect of intraocular pressure elevation in primate optic nerve. Arch Ophthalmol. 1979. 97:525–531.5. Yamamoto T, Kitazawa Y. Vascular pathogenesis of normal tension glaucoma: a possible pathogenetic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog Retin Eye Res. 1998. 17:127–143.6. Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999. 213:76–96.7. Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001. 85:1252–1259.8. Dreyer EB. A proposed role for excitotoxicity in glaucoma. J Glaucoma. 1998. 7:62–67.9. Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol. 1999. 43:142–150.10. Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984. 43:1369–1374.11. Pellegrini-Giampietro DE, Cherici G, Alesiani M, et al. Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage. J Neurosci. 1990. 10:1035–1041.12. Neal MJ, Cunningham JR, Hutson PH, Hogg J. Effects of ischaemia on neurotransmitter release from the isolated retina. J Neurochem. 1994. 62:1025–1033.13. Dreyer EB, Pan ZH, Storm S, Lipton SA. Greater sensitivity of larger retinal ganglion cells to NMDA-mediated cell death. Neuroreport. 1994. 5:629–631.14. Cazevieille C, Osborne NN. Retinal neurons containing kainate receptors are influenced by exogenous kainate and ischaemia while neurons lacking these receptors are not. Melatonin counteracts the effect of ischaemia and kainate. Brain Res. 1997. 755:91–100.15. Dutrait N, Culcasi M, Cazevieille C, et al. Calcium-dependent free radical generation in cultured retinal neurons injured by kainate. Neurosci Lett. 1995. 198:13–16.16. Nickells RW. Retinal ganglion cells death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996. 5:345–356.17. Snyder SH, Bredt DS. Biological roles of nitric oxide. Sci Am. 1992. 266:68–71. 74–77.18. Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994. 13:301–313.19. Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990. 347:768–770.20. Alonso D, Serrano J, Rodrigueg I, et al. Effects of oxygen and glucose deprivation on the expression and distribution of neuronal and inducible nitric oxide synthase and on protein nitration in rat cerebral cortex. J comp Neurol. 2002. 443:183–200.21. Ju WK, Kim KY, Park SJ, et al. Nitric oxide is involved in sustained and delayed cell death of rat retina following transient ischemia. Brain Res. 2000. 881:231–236.22. Hangai M, Yoshimura N, Hiroi K, et al. Inducible nitric oxide synthase in retinal ischemia-reperfusion injury. Exp Eye Res. 1996. 63:501–506.23. Ostwald P, Goldstein IM, Pachanda A, Roth S. Effect of nitric oxide synthase inhibition on blood flow after retinal ischemia in cats. Invest Ophthalmol Vis Sci. 1995. 36:2396–2403.24. Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002. 80:12–23.25. Grieb P, Rejdak R. Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J Neurosci Res. 2002. 67:143–148.26. Honjo M, Tanibara H, Kido N, et al. Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with NMDA- and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci. 2000. 41:552–560.27. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.28. Kang JH, Park KH, Kim YJ, et al. The neuroprotective effect of Ginexin on rat retinal ganglion cell in optic nerve crush injury model. J Korean Ophthalmol Soc. 2003. 44:965–970.29. Cheon EW, Park CH, Kang SS, et al. Nitric oxide synthase expression in the transient ischemic rat retina. neuroprotection of betaxolol. Neuroscience letters. 2002. 330:265–269.30. Yamauchi T, Kashii S, Yasuyoshi H, et al. Inhibition of glutamate-induced nitric oxide synthase activation by dopamine in cultured rat retinal neurons. Neuroscience letters. 2003. 347:155–158.31. Delbarre G, Delbarre B, Calnon F, Ferger A. Accumulation of amino acids and hydroxyl free radicals in brain and retina of gerbil after transient ischemia. J Ocul Pharmacol. 1991. 1:147–155.32. Perlman JI, McCole SM, Pulluru P, et al. Disturbance in the distribution of neurotransmitter in the rat retina after ischemia. Curr Eye Res. 1996. 15:589–596.33. Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. Arch Ophthalmol. 1957. 58:193–201.34. Onley JW. Brain lesion, obesity and other disturbances in mice treated with monosodium glutamate. Sciences. 1969. 164:719–721.35. Ehrlich D, Morgan IG. Kainic acid destroys displaced amacrine cells in post-hatch chicken retina. Neurosci Lett. 1981. 17:43–48.36. Morgan IG, Ingham CA. Kainic acid affects both plexiform layers of chicken retina. Neurosci Lett. 1981. 21:275–280.37. Siliprandi R, Canella R, Carmignoto G, et al. N-methyl D-aspartate induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992. 8:567–573.38. Campos EC, Schiavi C, Benedetti P, et al. Effect of citicoline on visual acuity in amblyopia: preliminary results. Graefes Arch Clin Exp Ophthalmol. 1995. 233:307–312.39. Rejdak R, Toczolowski J, Solski J, et al. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002. 34:146–149.40. Choi JS, Gwag BJ, Kim SJ, Joo CK. Effect of MK801 and CNQX on retinal injury induced by ischemia, NMDA, or kainate. J Korean Ophthalmol Soc. 1998. 39:1794–1800.41. Megumi H, Hidenobu T, Noriaki K, et al. Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with NMDA-and kainic acid-induced neuronal death. Inves Ophthalmol Vis Sci. 2000. 41:552–560.42. Brandstatter JH, Koulen P, Wassle H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 1998. 38:1385–1397.43. Iadecola C, Zhang F, Xu X. Role of nitric oxide synthase-containing vascular nerves in cerebrovasodilation elicited from cerebellum. Am J Physiol. 1993. 264:738–746.44. Biliar TR. Nitric oxide: novel biology with clinical relevance. Ann Surg. 1995. 221:339–349.45. Perez MT, Larsson B, Alm P, et al. Localization of neuronal nitric oxide synthase immunoreactivity in rat and rabbit retinas. Exp Brain Res. 1995. 104:207–217.46. Haverkamp S, Kolb H, Cuenca N. Endothelial nitric oxide synthase (eNOS) is localized to Muller cells in all vertebrate retinas. Vision Res. 1999. 39:2299–2303.47. Yamamoto R, Bredt DS, Snyder SH, Stone RA. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993. 54:189–200.48. Vorwerk CK, Hyman BT, Miller JW, et al. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Invest Ophthalmol Vis Sci. 1997. 38:2038–2044.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Etomidate on Kainic Acid-induced Neurotoxicity in Rat Hippocampus
- Effect of Etomidate on the Expressions of c-JUN and Heat Shock Protein 70 Induced by Kainic Acid in Rats
- Effect of Valproic Acid on Bcl-2, Bim, and Caspase-3 Expression in Rat Hippocampus after Kainic Acid-Induced Seizures
- Effect of Pioglitazone on Excitotoxic Neuronal Damage in the Mouse Hippocampus
- Citicoline Protects Against Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion