Korean J Ophthalmol.
2005 Sep;19(3):174-178. 10.3341/kjo.2005.19.3.174.
Therapeutic Effect of Umbilical Cord Serum Eyedrops for Persistent Corneal Epithelial Defect
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University College of Medicine, Gwangju, Korea. kcyoon@chonnam.ac.kr
- KMID: 754420
- DOI: http://doi.org/10.3341/kjo.2005.19.3.174
Abstract
- PURPOSE
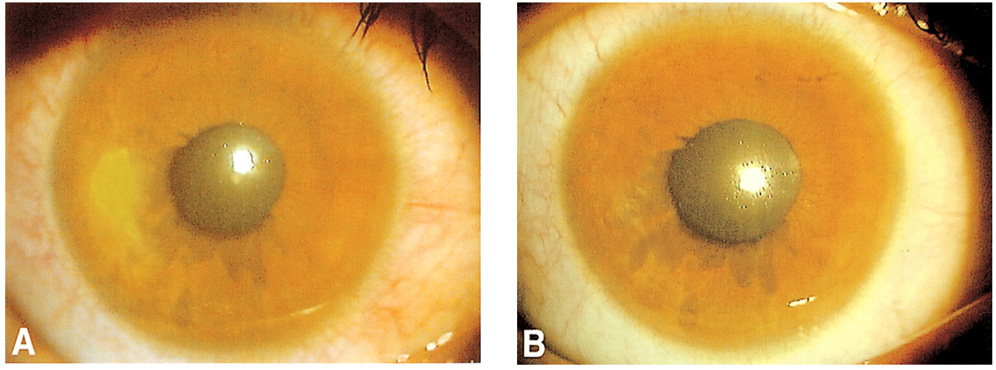
To evaluate the therapeutic effect of umbilical cord serum in the treatment of persistent epithelial defect of the cornea. METHODS: Fourteen eyes of 14 patients with persistent epithelial defect that had persisted for at least 2 weeks despite conventional treatment were treated with 20% umbilical cord serum eyedrops six times a day. The images of the epithelial defects were captured using a camera attached to a slit lamp biomicroscope and the areas of the epithelial defects were calculated. Treatment was considered effective for epithelial defect healing within 2 weeks, partially effective for healing within 2 to 4 weeks, and ineffective for healing requiring either more than 1 month or additional measures. RESULTS: Mean duration of epithelial defect before treatment was 7.2+/-6.3 weeks, and mean area was 7.86+/-7.32 mm2. Umbilical cord serum therapy was effective in 6 eyes (42.9%), partially effective in 6 (42.9%), and ineffective in 2 (14.2%). Nevertheless, the epithelial defects in both the ineffective eyes were eventually healed within 8 weeks. Mean healing time in effective or partially effective cases was 2.75+/-1.06 weeks. CONCLUSIONS: The use of umbilical cord serum eyedrops for the treatment of persistent epithelial defect is effective.
MeSH Terms
Figure
Cited by 2 articles
-
Use of Umbilical Cord Serum in Ophthalmology
Kyung Chul Yoon
Chonnam Med J. 2014;50(3):82-85. doi: 10.4068/cmj.2014.50.3.82.Effect of Combined Treatment with Cyclosporine A and Cord Serum for Dry Eye Associated with Graft-Versus-Host-Disease
Jung Han Choi, Han Jin Oh, Kyung Chul Yoon
J Korean Ophthalmol Soc. 2013;54(4):587-594. doi: 10.3341/jkos.2013.54.4.587.
Reference
-
1. Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eye and epithelial defect: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001. 85:1188–1197.2. Nishida T, Nakamura M, Ofuji K, et al. Synergic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996. 169:159–166.3. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004. 111:1115–1120.4. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984. 27:459–461.5. Tsubota K, Goto E, Shimmura S, et al. Treatment of persistent epithelial defect by autologous serum application. Ophthalmology. 1999. 106:1984–1989.6. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol. 1999. 83:390–395.7. Goto E, Shimmura S, Shimazaki J, Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001. 20:807–810.8. del Castillo JM, de la Casa JM, Sardina RC, et al. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002. 21:781–783.9. Borderie VM, Mourra N, Laroche L. Influence of fetal calf serum, fibroblast, growth factors, and hepatocyte growth factor on three-dimensional cultures of human keratocytes in collagen gel matrix. Graefes Arch Clin Exp Ophthalmol. 1999. 237:861–869.10. Vajpayee RB, Mukerji N, Tandon R, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003. 87:1312–1316.11. Mukerji N, Vajpayee RB, Sharma N. Technique of area measurement of epithelial defects. Cornea. 2003. 22:549–551.12. Ubels J, Loley K, Rismondo V. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci. 1986. 27:1261–1269.13. Ohashi Y, Motokura M, Kinoshita Y, et al. Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci. 1989. 30:1879–1887.14. Van Setten G, Tervo T, Tervo K, et al. Epidermal growth factor (EGF) in ocular fluid: presence, origin and therapeutic consideration. Acta Ophthalmol Suppl. 1992. 202:54–59.15. Noble BA, Loh RSK, MacLennan S, et al. Comparison of autologous serum eyedrops with conventional therapy in a randomized controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004. 88:647–652.16. Rodeck U, Jost M, Kari C, et al. EGF-R dependent regulation of keratinocyte survival. J Cell Sci. 1997. 110:113–132.17. Yashino Y, Garg R, Monroy D, et al. Production and secretion of transforming growth factor beta (TGF-β) by the human lacrimal gland. Curr Eye Res. 1996. 15:615–624.18. Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991. 53:753–758.19. Nishida T, Ohashi Y, Awata T, Manabe R. Fibronectin. A new therapy for corneal trophic ulcer. Arch Ophthalmol. 1983. 101:1046–1048.20. Phan TM, Foster CS, Boruchoff SA, et al. Topical fibronectin in the treatment of persistent corneal epithelial defects and trophic ulcers. Am J Ophthalmol. 1987. 104:494–501.21. Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000. 107:1347–1352.22. Nishida T, Nakamura M, Ofuji K, et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996. 169:159–166.23. Nishida T, Nakamura M, Murakami J, et al. Epidermal growth factor stimulates corneal epithelial cell attachment to fibronectin through a fibronectin receptor system. Invest Ophthalmol Vis Sci. 1992. 33:2464–2469.24. Singh G, Foster CS. Growth factors in treatment of non-healing corneal ulcer and recurrent erosions. Cornea. 1989. 8:45–53.25. Tsubota K, Higuchi A. Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin. 2000. 40:113–122.26. Tananuvat N, Daniell M, Suillivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001. 20:802–806.27. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eyedrops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003. 31:579–583.28. Yoo JW, Chung JH, Lee HR. Effects of topically applied autologous serum on experimental corneal epithelial healing following alkali wounds. J Korean Ophthalmol Soc. 1998. 39:2003–2023.29. Park DH, Yoon BJ. The anticollagenase effect of serum in alkali-burned cornea in rabbit. J Korean Ophthalmol Soc. 1988. 29:511–515.30. Lagnado R, King AJ, Donald F, Dua HS. A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br J Ophthalmol. 2004. 88:464–465.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Umbilical Cord Serum in Ophthalmology
- Clinical efficacy of topical homologous fibronectin in persistent corneal epithelial disorders
- Topical Autologous Fibronectin in the Treatment of Persistent Corneal Epithelial defects
- The Effect of 5% Serum Albumin on Intractable Corneal Epithelial Keratitis: a Case Series and Literature Review
- Therapeutic Effect of Topical Autologous Serum in Recurrent Punctate Corneal Erosion