Korean J Radiol.
2001 Sep;2(3):151-158. 10.3348/kjr.2001.2.3.151.
MR Imaging-Histopathologic Correlation of Radiofrequency Thermal Ablation Lesion in a Rabbit Liver Model: Observation during Acute and Chronic Stages
- Affiliations
-
- 1Department of Diagnostic Radiology, Chonbuk National University Medical School, Chonbuk, Korea. jmsh@chonbuk.ac.kr
- KMID: 754100
- DOI: http://doi.org/10.3348/kjr.2001.2.3.151
Abstract
OBJECTIVE
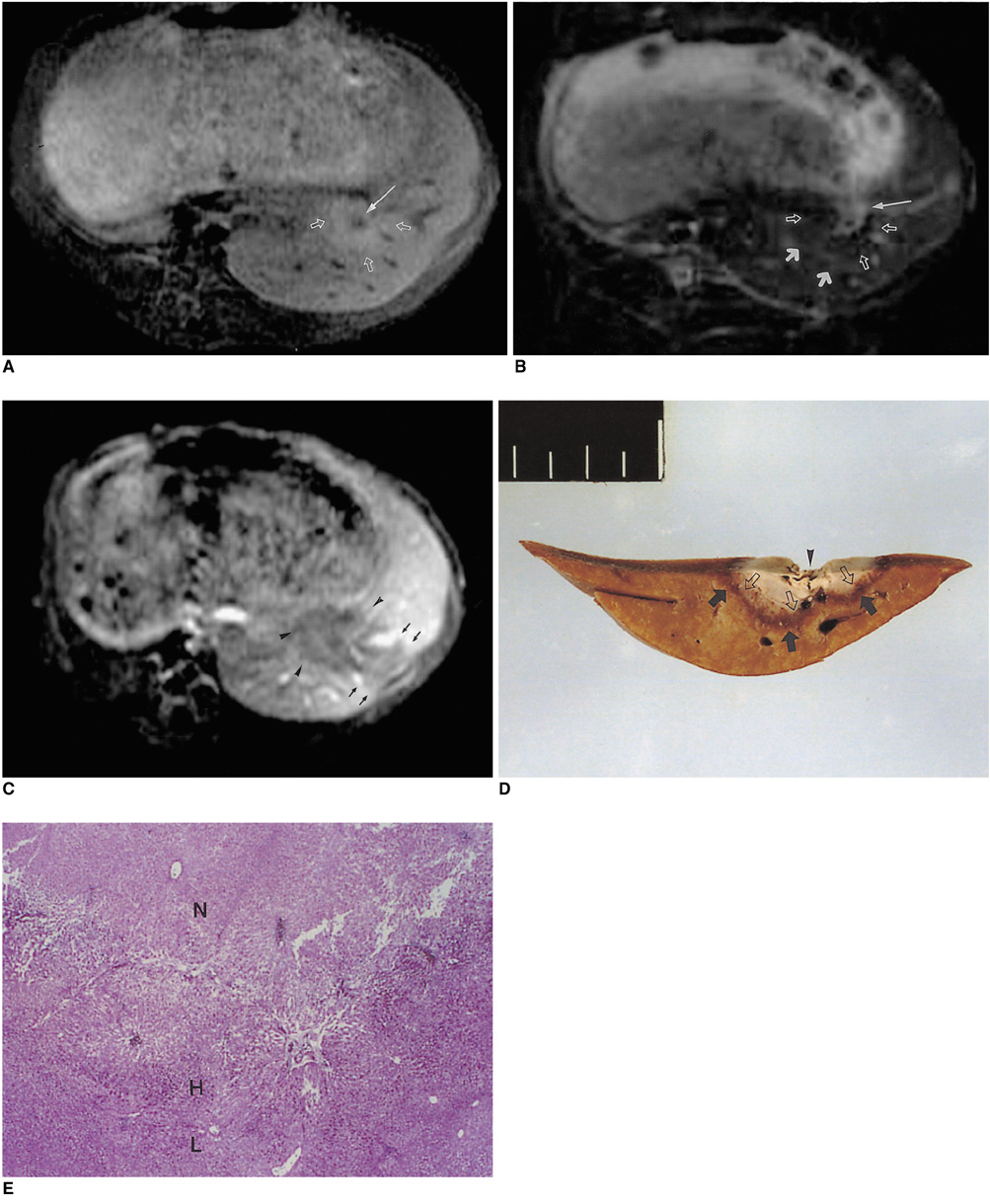
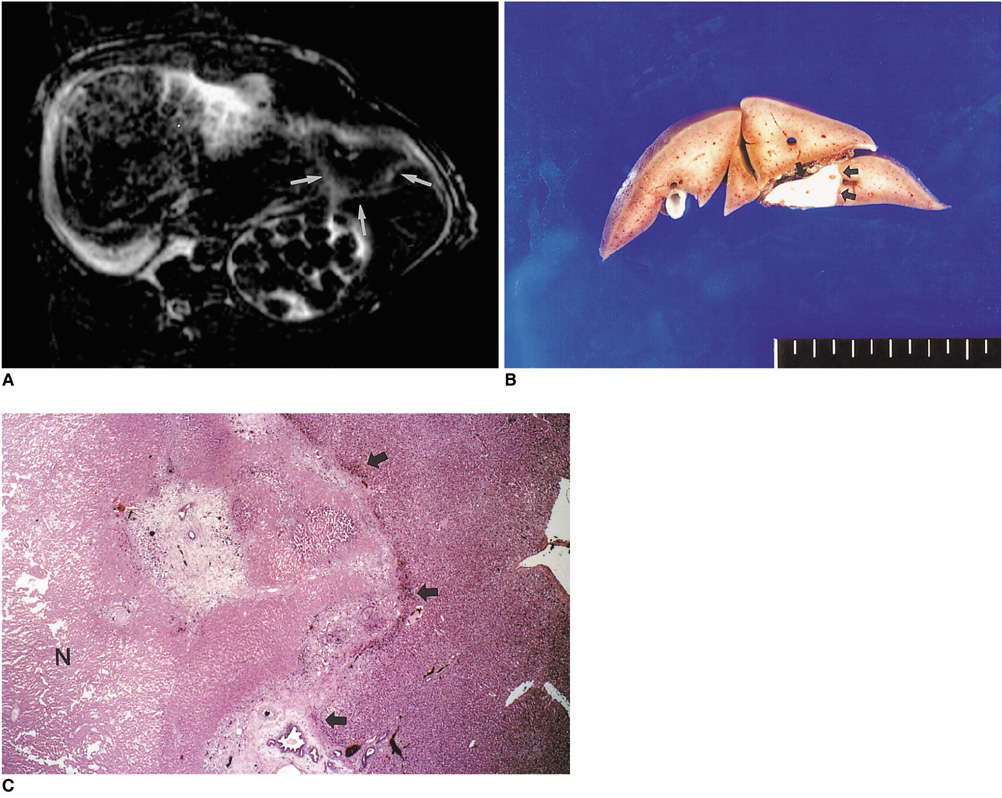
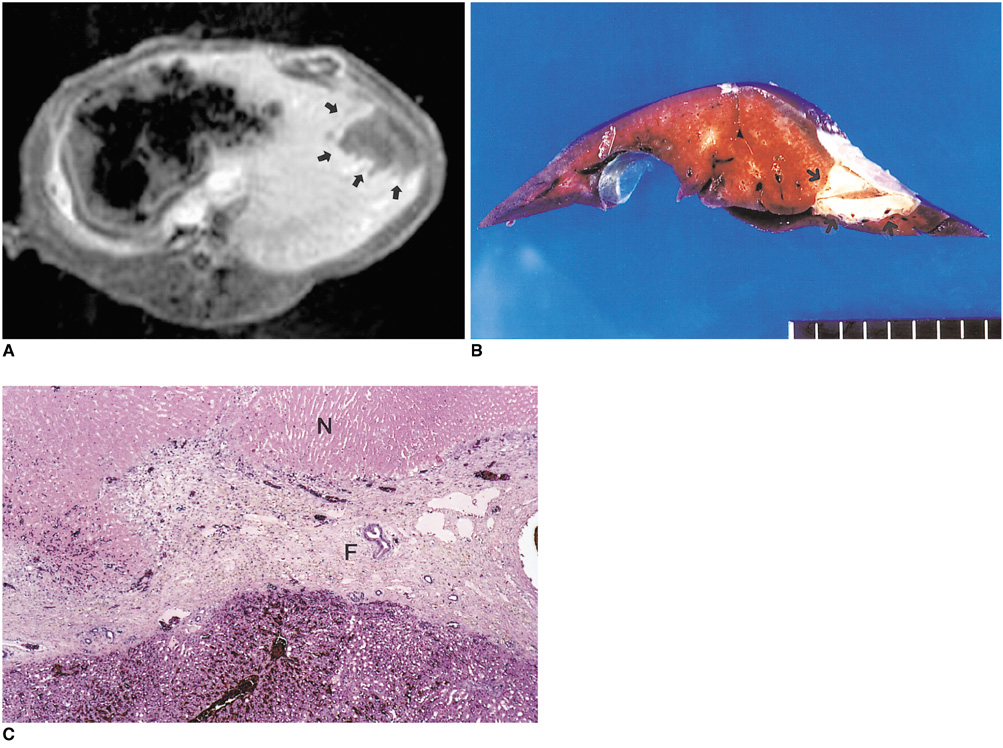
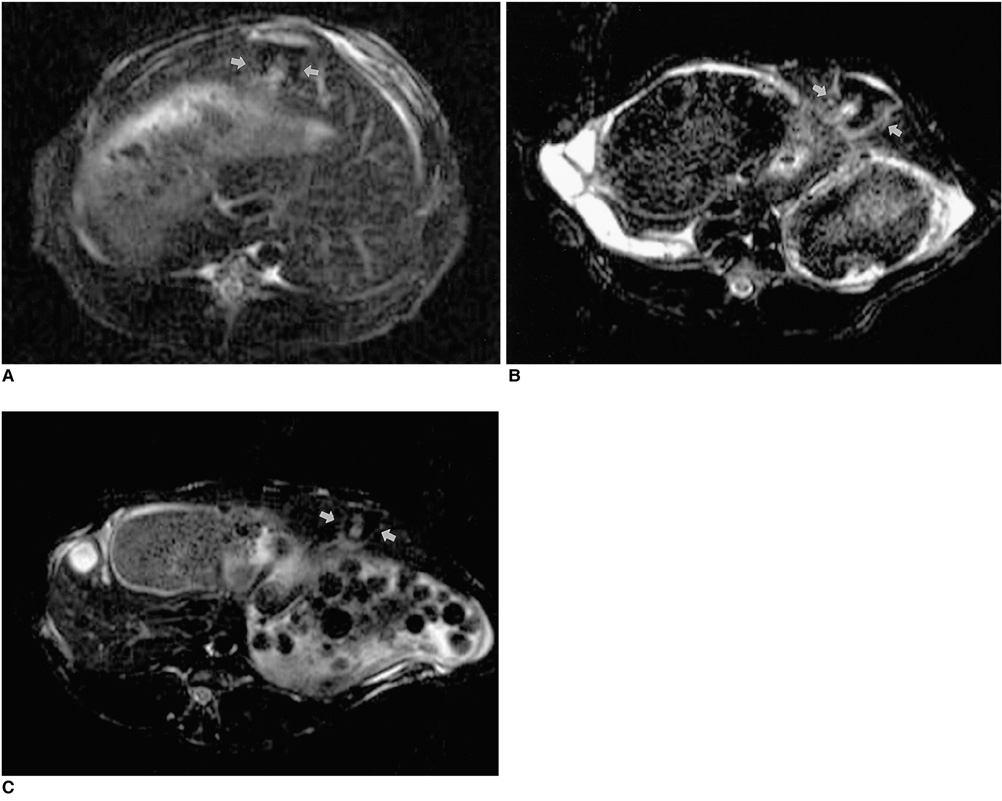
To determine the ability of MR imaging to detect the pathological changes occurring in radiofrequency (RF) thermal lesions and to assess its accuracy in revealing the extent of tissue necrosis. MATERIALS AND METHODS: Using an RF electrode, thermal lesions were created in the livers of 18 rabbits. The procedure involved three phases. In the acute phase, six animals were killed the day after performing thermal ablation with RF energy, and two on day 3. In the subacute and chronic phases, eight rabbits underwent percutaneous hepatic RF ablation. After performing MR imaging, two animals were sacrificed at 1, 2, 4, and 7 weeks after the procedure, and MR-pathologic correlation was performed. RESULTS: In the acute phase, the thermal ablation lesions appeared at gross examination as well-circumscribed, necrotic areas, representing early change in the coagulative necrosis seen at microscopic examination. They were hypointense on T2-weighted images, and hyperintense on T1-weighted images. Gadolinium-enhanced MR imaging showed that a thin hyperemic rim surrounded the central coagulative necrosis. In the subacute phase, ablated lesions also showed extensive coagulative necrosis and marked inflammation at microscopic examination. Beyond two weeks, the lesions showed gradual resorption of the necrotic area, with a peripheral fibrovascular rim. The size of lesions measured by MR imaging correlated well with the findings at gross pathologic examination. CONCLUSION: MR imaging effectively demonstrates the histopathological tissue change occurring after thermal ablation, and accurately determines the extent of the target area.
Figure
Cited by 1 articles
-
Moving-Shot versus Fixed Electrode Techniques for Radiofrequency Ablation: Comparison in an
Ex-Vivo Bovine Liver Tissue Model
Eun Ju Ha, Jung Hwan Baek, Jeong Hyun Lee
Korean J Radiol. 2014;15(6):836-843. doi: 10.3348/kjr.2014.15.6.836.
Reference
-
1. Sanctis JTD, Goldberg SN, Mueller PR. Percutaneous treatment of hepatic neoplasms; a review of current techniques. Cardiovasc Intervent Radiol. 1998. 21:273–296.2. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surgery. 2000. 232:381–391.3. Curley SA, Izzo F, Delrio P, Ellis LN, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surgery. 1999. 230:1–8.4. Vogl TJ, Muller PK, Hammerstingle R, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995. 196:257–265.5. Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994. 74:817–825.6. Goldberg SN, Livraghi T, Solbiati L, Gazelle GS. Gazelle GS, Saini S, Mueller PR, editors. In situ ablation of focal hepatic neoplasms. Hepatobiliary and pancreatic radiology: imaging and intervention. 1997. New York, NY: Thieme;470–502.7. Rossi S, Di Stasis M, Buscarini E, et al. Percutneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR. 1996. 167:759–768.8. Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radiofrequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997. 202:195–203.9. Goldberg SN, Gazelle GS, Solbiati L. Ablation of liver tumors using percutaneous RF therapy. AJR. 1998. 170:1023–1028.10. Solbiati L, Goldberg SN, Tiziana I, et al. Long-term follow-up of liver metastases treated with percutaneous US-guided radiofrequency (RF) ablation using internally-cooled electrodes (abstr). Radiology. 1998. 209(P):449.11. Livraghi T, Meloni F, Goldberg SN, Lazzaroni S, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.12. Siperstein A, Garland A, Engle K, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000. 7:106–113.13. Catalano O, Cusati B, Sandomenico F, Nunziata A, Siani A. Helical CT findings in patients with hepatocellular carcinoma treated with percutaneous ablation procedure. J Comput Assist Tomogr. 2000. 24:748–754.14. Sironi S, Livraghi T, Meloni F, Cobelli FD, Ferrero C, Maschio AD. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow-up. AJR. 1999. 173:1225–1229.15. Boaz TL, Lewin JS, Chung YC, Duerk JL, Clampitt ME, Haaga JR. MR monitoring of MR-guided radiofrequency thermal ablation of normal liver in an animal model. J Magn Reson Imaging. 1998. 8:64–69.16. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignacy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR. 2000. 174:323–331.17. Sanchez R, vanSonnenberg E, D'Agostino H, et al. Percutaneous tissue ablation by radiofrequency thermal energy as a prelim to tumor ablation. Min Inv Ther. 1993. 2:299–305.18. Curley SA, Davidson BS, Fleming RY, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc. 1997. 11:729–733.19. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR. 1996. 167:759–768.20. Solbiati L, Goldberg SN, Ierace T, et al. Radiofrequency ablation of hepatic metastases: postprocedural assessment with a US microbubble contrast agent-early experience. Radiology. 1999. 211:643–649.21. Steiner P, Botnar R, Goldberg SN, Gazelle GS, Debatin JF. Monitoring of radiofrequency tissue ablation in an interventional MR environment: preliminary ex vivo and in vivo results. Invest Radiol. 1997. 32:671–678.22. Fullerton G, Stark D, Bradley W. Physiologic basis of magnetic relaxation. Magnetic resonance imaging. 1992. 2nd ed. St. Louis: Mosby Year Book;95–99.23. Darkazanili A, Hynynen K, Unger E, Schenck J. On-line monitoring of ultrasonic surgery with MR imaging. J Magn Reson Imaging. 1993. 3:509–514.24. McGahan JP, Brock JM, Tesluk H, et al. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992. 3:291–297.25. Raman SS, Lu DSK, Vodopich DJ, Sayre J, Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopathology. AJR. 2000. 175:1253–1258.26. Castro DJ, Abergel PR, Johnston KJ, et al. Wound healing: biological effects of Nd:YAG laser on collagen metabolism in pig skin in comparison to thermal burn. Ann Plast Surg. 1983. 11:131–140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Comparison of Lesion Conspicuity of Radiofrequency Ablation Zones among MR Sequences According to Time in the Normal Rabbit Liver
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention
- Ferucarbotran-Enhanced MR Imaging of the Radiofrequency Ablation Zones in the Normal Rabbit Liver: Comparison with the Conventional MR Imaging