Yonsei Med J.
2008 Jun;49(3):422-428. 10.3349/ymj.2008.49.3.422.
Investigation of the Cause of Readmission to the Intensive Care Unit for Patients with Lung Edema or Atelectasis
- Affiliations
-
- 1Department of Emergency and Critical Care Medicine, School of Medicine, Kyushu University, Fukuoka, Japan. yoshinori216@h2.dion.ne.jp
- KMID: 724258
- DOI: http://doi.org/10.3349/ymj.2008.49.3.422
Abstract
- PURPOSE
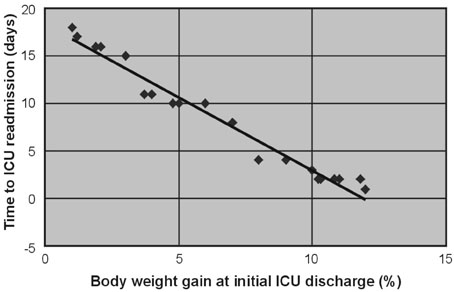
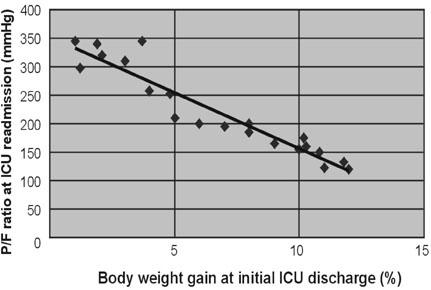
For patients with acute respiratory failure due to lung edema or atelectasis, Surplus lung water that is not removed during an initial stay in the Intensive Care Unit (ICU) may be related to early ICU readmission. Therefore, we performed a retrospective study of patient management during the first ICU stay for such patients. MATERIALS AND METHODS: Of 1,835 patients who were admitted to the ICU in the 36 months from January, 2003 to December, 2005, 141 were patients readmitted, and the reason for readmission was lung edema or atelectasis in 21 patients. For these 21 patients, correlations were investigated between body weight gain at the time of initial ICU discharge (weight upon discharge from the ICU ÷ weight when entering the ICU) and the time to ICU readmission, between body weight gain and the P/F ratio at ICU readmission, between the R/E ratio (the period using a respirator (R) ÷ the length of the ICU stay after extubation (E)) and the time to ICU readmission, between the R/E ratio and body weight gain, and between body weight gain until extubation and the time to extubation. RESULTS: A negative linear relationship was found between body weight gain at the time of initial ICU discharge and the time to ICU readmission, and between body weight gain at the time of ICU discharge and the P/F ratio at ICU readmission. If body weight had increased by more than 10% at ICU discharge or the P/F ratio was below 150, readmission to the ICU within three days was likely. Patients with a large R/E ratio, a large body weight gain, and a worsening P/F ratio immediately after ICU discharge were likely to be readmitted soon to the ICU. Loss of body weight during the period of respirator support led to early extubation, since a positive correlation was found between the time to extubation and body weight gain. CONCLUSION: Fluid management failure during the first ICU stay might cause ICU readmission for patients who had lung edema or atelectasis. Therefore, a key to the prevention of ICU readmission is to ensure complete recovery from lung failure before the initial ICU discharge. Strict water management is crucial based on body weight measurement and removal of excess lung water is essential. In addition, an apparent improvement in respiratory state may be due to respiratory support, and such an improvement should be viewed cautiously. Loss of weight at the refilling stage of transfusion prevents ICU readmission and may decrease the length of the ICU stay.
MeSH Terms
Figure
Cited by 2 articles
-
Readmission risk factors for children admitted to pediatric intensive care unit with respiratory tract disease
Woo Jin Chung, Da Hye Yoon, Eui Gyung Lee, Kyong Won Bang, Hwan Su Kim, Yoon Hong Chun, Jong-Seo Yoon, Hyun Hee Kim, Jin Tack Kim, Joon Sung Lee
Allergy Asthma Respir Dis. 2014;2(2):128-133. doi: 10.4168/aard.2014.2.2.128.What intensive care unit readmission means
Joongbum Cho
Allergy Asthma Respir Dis. 2014;2(2):83-84. doi: 10.4168/aard.2014.2.2.83.
Reference
-
1. Forbes GB. Weight loss during fasting: implications for the obese. Am J Clin Nutr. 1970. 23:1212–1219.
Article2. Benedict FG. A study of Prolonged Fasting. 1915. Washington, DC: Carnegie Institution of Washington;69–87.3. Drenick EJ, Swendseid ME, Blahd WH, Tuttle SG. Prolonged starvation as treatment for severe obesity. JAMA. 1964. 187:100–105.
Article4. Spady DW, Payne PR, Picou D, Waterlow JC. Energy balance during recovery from malnutrition. Am J Clin Nutr. 1976. 29:1073–1088.
Article5. Long CL. Energy balance and carbohydrate metabolism in infection and sepsis. Am J Clin Nutr. 1977. 30:1301–1310.6. Long CL, Kinney JM, Geiger JW. Nonsuppressability of gluconeogenesis by glucose in septic patients. Metabolism. 1976. 25:193–201.
Article7. Zaitsu A. The latest trends in the treatment of acute respiratory failure. Fukuoka Igaku Zasshi. 1990. 81:391–395.8. Covelli HD, Nessan VJ, Tuttle WK 3rd. Oxygen derived variables in acute respiratory failure. Crit Care Med. 1983. 11:646–649.
Article9. Nakae H, Endo S, Inada K, Kasai T, Yoshida M. Significance of alpha-tocopherol and interleukin 8 in septic adult respiratory distress syndrome. Res Commun Chem Pathol Pharmacol. 1994. 84:197–202.10. Endo S, Inada K, Inoue Y, Kuwata Y, Suzuki M, Yamashita H, et al. Two types of septic shock classified by the plasma levels of cytokines and endotoxin. Circ Shock. 1992. 38:264–274.11. Endo S, Inada K, Yamada Y, Kasai T, Takakuwa T, Nakae H, et al. Plasma tumour necrosis factor-alpha (TNF-alpha) levels in patients with burns. Burns. 1993. 19:124–127.
Article12. Endo S, Inada K, Yamashita H, Takakuwa T, Nakae H, Kasai T, et al. Platelet-activating factor (PAF) acetylhydrolase activity, type II phospholipase A2, and cytokine levels in patients with sepsis. Res Commun Chem Pathol Pharmacol. 1994. 83:289–295.13. Endo S, Inada K, Ceska M, Takakuwa T, Yamada Y, Nakae H, et al. Plasma interleukin 8 and polymorphonuclear leukocyte elastase concentrations in patients with septic shock. J Inflamm. 1995. 45:136–142.14. Petty TL. Protease mechanisms in the pathogenesis of acute lung injury. Ann N Y Acad Sci. 1991. 624:267–277.
Article15. Edens HA, Parkos CA. Neutrophil transendothelial migration and alteration in vascular permeability: focus on neutrophil-derived azurocidin. Curr Opin Hematol. 2003. 10:25–30.
Article16. Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005. 175:2598–2605.
Article17. Guo M, Wu MH, Granger HJ, Yuan SY. Focal adhesion kinase in neutrophil-induced microvascular hyperpermeability. Microcirculation. 2005. 12:223–232.
Article18. Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986. 133:913–927.19. Imm A, Carlson RW. Fluid resuscitation in circulatory shock. Crit Care Clin. 1993. 9:313–333.
Article20. Falk JL, O'Brien JF, Kerr R. Fluid resuscitation in traumatic hemorrhagic shock. Crit Care Clin. 1992. 8:323–340.
Article21. Domsky MF, Wilson RF. Hemodynamic resuscitation. Crit Care Clin. 1993. 9:715–726.
Article22. Dantzker DR. Dantzker DR, editor. Pulmonary gas exchange. Cardiopulmonary critical care. 1991. 2nd ed. Philadelphia: W.B. Saunders;25–43.23. Lanken PN. Grippi MA, editor. Ventilation-perfusion relationships. Pulmonary pathophysiology. 1995. Philadelphia: JB Lippincott;195–210.24. Buohuys A. Fenn WO, Rahn H, editors. Respiratory dead space. Handbook of physiology: respiration. 1964. Bethesda, MD: American Physiological Society;699–714.25. D'Alonzo GE, Dantzker DR. Respiratory failure, mechanisms of abnormal gas exchange, and oxygen delivery. Med Clin North Am. 1983. 67:557–571.26. Matsui M, Kudo T, Kudo M, Ishihara H, Matsuki A. The endocrine response after burns. Agressologie. 1991. 32:233–235.27. Meduri GU, Mauldin GL, Wunderink RG, Leeper KV Jr, Jones CB, Tolley E, et al. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994. 106:221–235.
Article28. Bates JH, Campbell GD, Barron AL, McCracken GA, Morgan PN, Moses EB, et al. Microbial etiology of acute pneumonia in hospitalized patients. Chest. 1992. 101:1005–1012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atelectasis and the Risk Factors in the Patients Admitted to Pediatric Intensive Care Unit
- Comment on “Risk factors for intensive care unit readmission after lung transplantation: a retrospective cohort study”
- The Use of Lung Ultrasound in a Surgical Intensive Care Unit
- Risk factors for intensive care unit readmission after lung transplantation: a retrospective cohort study
- Discharge Decision-Making by Intensivists on Readmission to the Intensive Care Unit