Korean Circ J.
2023 Apr;53(4):239-250. 10.4070/kcj.2022.0312.
Antiarrhythmic Effect of Artemisinin in an Ex-vivo Model of Brugada Syndrome Induced by NS5806
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea
- 2Department of Cardiology, Kwangju Christian Hospital, Gwangju, Korea
- 3Division of Cardiology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- 4Department of Cardiovascular Medicine, Chonnam National University Hospital, Gwangju, Korea
- KMID: 2542294
- DOI: http://doi.org/10.4070/kcj.2022.0312
Abstract
- Background and Objectives
Brugada syndrome (BrS) is an inherited arrhythmia syndrome that presents as sudden cardiac death (SCD) without structural heart disease. One of the mechanisms of SCD has been suggested to be related to the uneven dispersion of transient outward potassium current (Ito ) channels between the epicardium and endocardium, thus inducing ventricular tachyarrhythmia. Artemisinin is widely used as an antimalarial drug. Its antiarrhythmic effect, which includes suppression of Ito channels, has been previously reported. We investigated the effect of artemisinin on the suppression of electrocardiographic manifestations in a canine experimental model of BrS.
Methods
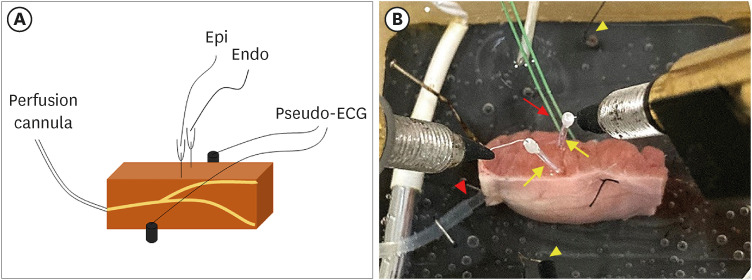
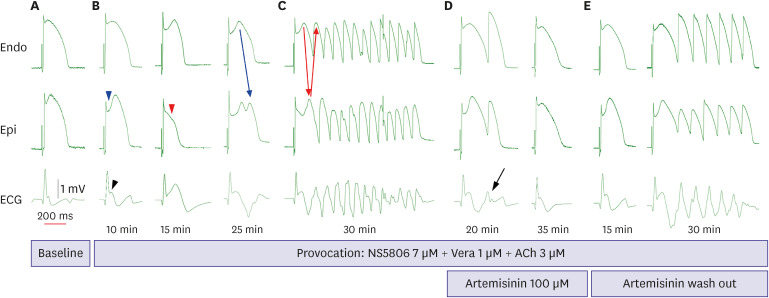
Transmural pseudo-electrocardiograms and epicardial/endocardial transmembrane action potentials (APs) were recorded from coronary-perfused canine right ventricular wedge preparations (n=8). To mimic the BrS phenotypes, acetylcholine (3 μM), calcium channel blocker verapamil (1 μM), and Ito agonist NS5806 (6–10 μM) were used. Artemisinin (100–150 μM) was then perfused to ameliorate the ventricular tachyarrhythmia in the BrS models.
Results
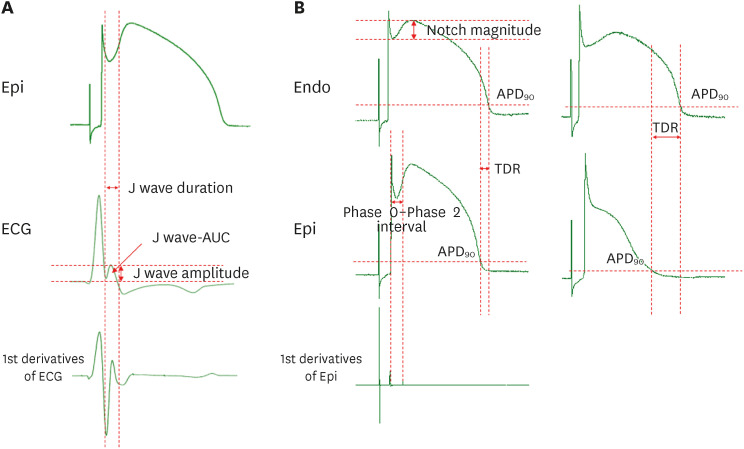
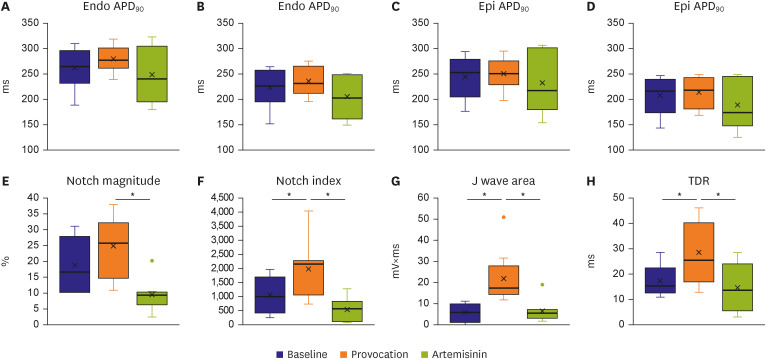
The provocation agents induced prominent J waves in all the models on the pseudoelectrocardiograms. The epicardial AP dome was attenuated. Ventricular tachyarrhythmia was induced in six out of 8 preparations. Artemisinin suppressed ventricular tachyarrhythmia in all 6 of these preparations and recovered the AP dome of the right ventricular epicardium in all preparations (n=8). J wave areas and epicardial notch indexes were also significantly decreased after artemisinin perfusion.
Conclusions
Our findings suggest that artemisinin has an antiarrhythmic effect on wedge preparation models of BrS. It might work by inhibition of potassium channels including Ito channels, subsequently suppressing ventricular tachycardia/ventricular fibrillation.
Figure
Cited by 1 articles
-
Can Artemisinin Be a Game Changer Even as an Antiarrhythmic Drug?
Jongmin Hwang
Korean Circ J. 2023;53(4):251-253. doi: 10.4070/kcj.2023.0031.
Reference
-
1. Brugada J, Campuzano O, Arbelo E, Sarquella-Brugada G, Brugada R. Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018; 72:1046–1059. PMID: 30139433.3. Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006; 29:1130–1159. PMID: 17038146.
Article4. Szél T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J Am Coll Cardiol. 2014; 63:2037–2045. PMID: 24657694.
Article5. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018; 138:e272–e391. PMID: 29084731.6. Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022; 43:3997–4126. PMID: 36017572.7. Yang BF, Li YR, Xu CQ, et al. Mechanisms of artemisinin antiarrhythmic action. Chin J Pharm Toxicol. 1999; 13:169–175.8. Yang BF, Luo DL, Bao LH, Zhang YC, Wang HZ. Artemisinin blocks activating and slowly activating K+ current in guinea pig ventricular myocytes. Acta Pharmacologica Sinica. 1998; 19:269–272. PMID: 10375742.9. So EC, Wu SN, Wu PC, Chen HZ, Yang CJ. Synergistic inhibition of delayed rectifier K+ and voltage-gated Na+ currents by artemisinin in pituitary tumor (GH3) cells. Cell Physiol Biochem. 2017; 41:2053–2066. PMID: 28456794.
Article10. Qiao G, Li S, Yang B, Li B. Inhibitory effects of artemisinin on voltage-gated ion channels in intact nodose ganglion neurones of adult rats. Basic Clin Pharmacol Toxicol. 2007; 100:217–224. PMID: 17371525.
Article11. Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013; 54:53–64. PMID: 23142540.
Article12. Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006; 113:1393–1400. PMID: 16534004.
Article13. Gurabi Z, Koncz I, Patocskai B, Nesterenko VV, Antzelevitch C. Cellular mechanism underlying hypothermia-induced ventricular tachycardia/ventricular fibrillation in the setting of early repolarization and the protective effect of quinidine, cilostazol, and milrinone. Circ Arrhythm Electrophysiol. 2014; 7:134–142. PMID: 24429494.
Article14. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999; 100:1660–1666. PMID: 10517739.
Article15. Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010; 49:543–553. PMID: 20659475.16. Yoon N, Jeong HK, Lee KH, Park HW, Cho JG. Right ventricular longitudinal conduction delay in patients with Brugada syndrome. J Korean Med Sci. 2021; 36:e75. PMID: 33754508.
Article17. Lambiase PD, Ahmed AK, Ciaccio EJ, et al. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation. 2009; 120:106–117. 1–104. PMID: 19564561.18. Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011; 123:1270–1279. PMID: 21403098.
Article19. Malhi N, Cheung CC, Deif B, et al. Challenge and impact of quinidine access in sudden death syndromes: a national experience. JACC Clin Electrophysiol. 2019; 5:376–382. PMID: 30898241.20. Andorin A, Gourraud JB, Mansourati J, et al. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 2017; 14:1147–1154. PMID: 28411139.
Article21. Behr ER, Ben-Haim Y, Ackerman MJ, Krahn AD, Wilde AA. Brugada syndrome and reduced right ventricular outflow tract conduction reserve: a final common pathway? Eur Heart J. 2021; 42:1073–1081. PMID: 33421051.
Article22. Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985; 228:1049–1055. PMID: 3887571.
Article23. Di Diego JM, Sun ZQ, Antzelevitch C. I(to) and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996; 271:H548–H561. PMID: 8770096.
Article24. Di Diego JM, Patocskai B, Barajas-Martinez H, et al. Acacetin suppresses the electrocardiographic and arrhythmic manifestations of the J wave syndromes. PLoS One. 2020; 15:e0242747. PMID: 33232375.
Article25. Giudicessi JR, Ye D, Tester DJ, et al. Transient outward current Ito gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm. 2011; 8:1024–1032. PMID: 21349352.
Article26. Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990; 67:615–627. PMID: 2397572.
Article27. Szél T, Koncz I, Antzelevitch C. Cellular mechanisms underlying the effects of milrinone and cilostazol to suppress arrhythmogenesis associated with Brugada syndrome. Heart Rhythm. 2013; 10:1720–1727. PMID: 23911896.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 Vaccination-Induced Ventricular Fibrillation in an Afebrile Patient With Brugada Syndrome
- A Case of Idiopathic Brugada ECG Pattern Developed in Relation To Fever
- Can Artemisinin Be a Game Changer Even as an Antiarrhythmic Drug?
- Anesthesia in a Patient with Brugada Syndrome without a Characteristic ECG Pattern: A case report
- Brugada Syndrome Presenting With Convulsion in the Emergency Department: A Case Report