Endocrinol Metab.
2023 Apr;38(2):190-202. 10.3803/EnM.2022.1599.
The Physiological Functions and Polymorphisms of Type II Deiodinase
- Affiliations
-
- 1Department of Histology and Embryology, School of Basic Medical Sciences, Southwest Medical University, China
- 2Key Laboratory of Medical Electrophysiology of Ministry of Education and Medical Electrophysiological Key Laboratory of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, China
- KMID: 2541875
- DOI: http://doi.org/10.3803/EnM.2022.1599
Abstract
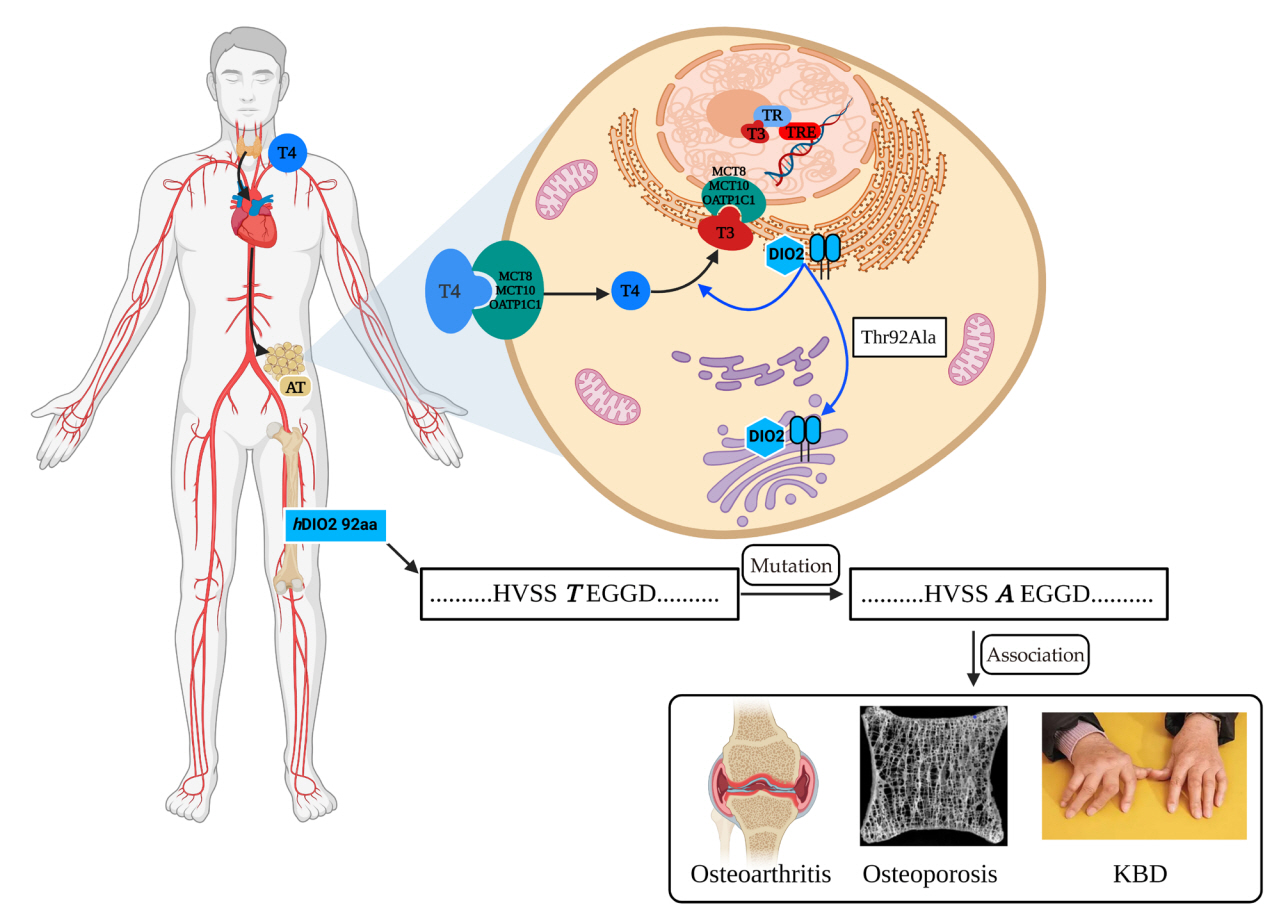
- Type II deiodinase (DIO2) is thought to provide triiodothyronine (T3) to the nucleus to meet intracellular needs by deiodinating the prohormone thyroxine. DIO2 is expressed widely in many tissues and plays an important role in a variety of physiological processes, such as controlling T3 content in developing tissues (e.g., bone, muscles, and skin) and the adult brain, and regulating adaptive thermogenesis in brown adipose tissue (BAT). However, the identification and cloning of DIO2 have been challenging. In recent years, several clinical investigations have focused on the Thr92Ala polymorphism, which is closely correlated with clinical syndromes such as type 2 diabetes, obesity, hypertension, and osteoarthritis. Thr92Ala-DIO2 was also found to be related to bone and neurodegenerative diseases and tumors. However, relatively few reviews have synthesized research on individual deiodinases, especially DIO2, in the past 5 years. This review summarizes current knowledge regarding the physiological functions of DIO2 in thyroid hormone signaling and adaptive thermogenesis in BAT and the brain, as well as the associations between Thr92Ala-DIO2 and bone and neurodegenerative diseases and tumors. This discussion is expected to provide insights into the physiological functions of DIO2 and the clinical syndromes associated with Thr92Ala-DIO2.
Keyword
Figure
Reference
-
1. Luongo C, Dentice M, Sal vatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol. 2019; 15:479–88.
Article2. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013; 1830:3937–45.
Article3. St Germain DL, Galton VA. The deiodinase family of selenoproteins. Thyroid. 1997; 7:655–68.
Article4. Maia AL, Berry MJ, Sabbag R, Harney JW, Larsen PR. Structural and functional differences in the dio1 gene in mice with inherited type 1 deiodinase deficiency. Mol Endocrinol. 1995; 9:969–80.
Article5. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002; 23:38–89.
Article6. Van der Geyten S, Segers I, Gereben B, Bartha T, Rudas P, Larsen PR, et al. Transcriptional regulation of iodothyronine deiodinases during embryonic development. Mol Cell Endocrinol. 2001; 183:1–9.
Article7. Lopez-Espindola D, Garcia-Aldea A, Gomez de la Riva I, Rodriguez-Garcia AM, Salvatore D, Visser TJ, et al. Thyroid hormone availability in the human fetal brain: novel entry pathways and role of radial glia. Brain Struct Funct. 2019; 224:2103–19.
Article8. Takemura Y, Yamaguchi S, Aoki N, Miura M, Homma KJ, Matsushima T. Gene expression of Dio2 (thyroid hormone converting enzyme) in telencephalon is linked with predisposed biological motion preference in domestic chicks. Behav Brain Res. 2018; 349:25–30.
Article9. Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982; 306:23–32.10. Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxinemediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006; 147:1735–43.
Article11. Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000; 97:1287–92.
Article12. Marsili A, Tang D, Harney JW, Singh P, Zavacki AM, Dentice M, et al. Type II iodothyronine deiodinase provides intracellular 3,5,3’-triiodothyronine to normal and regenerating mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2011; 301:E818–24.
Article13. Bomer N, Pavez-Giani MG, Deiman FE, Linders AN, Hoes MF, Baierl CL, et al. Selenoprotein DIO2 is a regulator of mitochondrial function, morphology and UPRmt in human cardiomyocytes. Int J Mol Sci. 2021; 22:11906.
Article14. Bassett JH, Boyde A, Howell PG, Bassett RH, Galliford TM, Archanco M, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci U S A. 2010; 107:7604–9.
Article15. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001; 108:1379–85.
Article16. Diez D, Morte B, Bernal J. Single-cell transcriptome profiling of thyroid hormone effectors in the human fetal neocortex: expression of SLCO1C1, DIO2, and THRB in specific cell types. Thyroid. 2021; 31:1577–88.
Article17. Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010; 120:2206–17.
Article18. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008; 29:898–938.
Article19. Gao Y, Zhao L, Son JS, Liu X, Chen Y, Deavila JM, et al. Maternal exercise before and during pregnancy facilitates embryonic myogenesis by enhancing thyroid hormone signaling. Thyroid. 2022; 32:581–93.
Article20. Straczkowski M, Nikolajuk A, Stefanowicz M, Matulewicz N, Fernandez-Real JM, Karczewska-Kupczewska M. Adipose tissue and skeletal muscle expression of genes associated with thyroid hormone action in obesity and insulin resistance. Thyroid. 2022; 32:206–14.
Article21. Kojima Y, Kondo Y, Fujishita T, Mishiro-Sato E, Kajino-Sakamoto R, Taketo MM, et al. Stromal iodothyronine deiodinase 2 (DIO2) promotes the growth of intestinal tumors in ApcΔ716 mutant mice. Cancer Sci. 2019; 110:2520–8.
Article22. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018; 24:39–49.
Article23. Meyer EL, Goemann IM, Dora JM, Wagner MS, Maia AL. Type 2 iodothyronine deiodinase is highly expressed in medullary thyroid carcinoma. Mol Cell Endocrinol. 2008; 289:16–22.
Article24. Kim BW, Daniels GH, Harrison BJ, Price A, Harney JW, Larsen PR, et al. Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J Clin Endocrinol Metab. 2003; 88:594–8.
Article25. Tannahill LA, Visser TJ, McCabe CJ, Kachilele S, Boelaert K, Sheppard MC, et al. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours. Clin Endocrinol (Oxf). 2002; 56:735–43.
Article26. Nauman P, Bonicki W, Michalik R, Warzecha A, Czernicki Z. The concentration of thyroid hormones and activities of iodothyronine deiodinases are altered in human brain gliomas. Folia Neuropathol. 2004; 42:67–73.27. Zhou Z, Wang H, Zhang X, Song M, Yao S, Jiang P, et al. Defective autophagy contributes to endometrial epithelialmesenchymal transition in intrauterine adhesions. Autophagy. 2022; 18:2427–42.
Article28. Ma SF, Xie L, Pino-Yanes M, Sammani S, Wade MS, Letsiou E, et al. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol. 2011; 45:1203–11.
Article29. An X, Ogawa-Wong A, Carmody C, Ambrosio R, Cicatiello AG, Luongo C, et al. A type 2 deiodinase-dependent increase in Vegfa mediates myoblast-endothelial cell cross-talk during skeletal muscle regeneration. Thyroid. 2021; 31:115–27.
Article30. Adu-Gyamfi EA, Lamptey J, Chen XM, Li FF, Li C, Ruan LL, et al. Iodothyronine deiodinase 2 (DiO2) regulates trophoblast cell line cycle, invasion and apoptosis; and its downregulation is associated with early recurrent miscarriage. Placenta. 2021; 111:54–68.
Article31. Arnaldi LA, Borra RC, Maciel RM, Cerutti JM. Gene expression profiles reveal that DCN, DIO1, and DIO2 are underexpressed in benign and malignant thyroid tumors. Thyroid. 2005; 15:210–21.
Article32. Murakami M, Araki O, Morimura T, Hosoi Y, Mizuma H, Yamada M, et al. Expression of type II iodothyronine deiodinase in brain tumors. J Clin Endocrinol Metab. 2000; 85:4403–6.33. Davey JC, Becker KB, Schneider MJ, St Germain DL, Galton VA. Cloning of a cDNA for the type II iodothyronine deiodinase. J Biol Chem. 1995; 270:26786–9.
Article34. Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase: a selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996; 98:405–17.
Article35. Araki O, Murakami M, Morimura T, Kamiya Y, Hosoi Y, Kato Y, et al. Assignment of type II iodothyronine deiodinase gene (DIO2) to human chromosome band 14q24.2-->q24.3 by in situ hybridization. Cytogenet Cell Genet. 1999; 84:73–4.36. Buettner C, Harney JW, Larsen PR. The role of selenocysteine 133 in catalysis by the human type 2 iodothyronine deiodinase. Endocrinology. 2000; 141:4606–12.
Article37. Callebaut I, Curcio-Morelli C, Mornon JP, Gereben B, Buettner C, Huang S, et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem. 2003; 278:36887–96.
Article38. Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005; 7:698–705.
Article39. Zavacki AM, Drigo RAE, Freitas BC, Chung M, Harney JW, Egri P, et al. The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol Cell Biol. 2009; 29:5339–47.
Article40. Drigo RAE, Bianco AC. Type 2 deiodinase at the crossroads of thyroid hormone action. Int J Biochem Cell Biol. 2011; 43:1432–41.
Article41. Zeold A, Pormuller L, Dentice M, Harney JW, Curcio-Morelli C, Tente SM, et al. Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J Biol Chem. 2006; 281:31538–43.
Article42. Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000; 141:4309–12.
Article43. McAninch EA, Jo S, Preite NZ, Farkas E, Mohacsik P, Fekete C, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015; 100:920–33.
Article44. Bianco AC, Kim BS. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes. 2018; 25:341–6.
Article45. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017; 390:1550–62.
Article46. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014; 24:1670–751.
Article47. Rastoldo G, Marouane E, El-Mahmoudi N, Pericat D, Watabe I, Lapotre A, et al. L-thyroxine improves vestibular compensation in a rat model of acute peripheral vestibulopathy: cellular and behavioral aspects. Cells. 2022; 11:684.
Article48. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020; 105:e3090–104.
Article49. Williams GR, Bassett JH. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. J Endocrinol. 2011; 209:261–72.50. Shakir MK, Brooks DI, McAninch EA, Fonseca TL, Mai VQ, Bianco AC, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021; 106:e4400–13.
Article51. Ahmed ZS, Sherin RP, Fonseca TL, Hoang TD, Shakir MK. Improvement of depression in a patient with hypothyroidism and deiodinase polymorphism with LT3 Therapy. Clin Case Rep. 2022; 10:e05651.
Article52. Wolff TM, Dietrich JW, Muller MA. Optimal hormone replacement therapy in hypothyroidism: a model predictive control approach. Front Endocrinol (Lausanne). 2022; 13:884018.53. Maino F, Cantara S, Forleo R, Pilli T, Castagna MG. Clinical significance of type 2 iodothyronine deiodinase polymorphism. Expert Rev Endocrinol Metab. 2018; 13:273–7.
Article54. Drigo RAE, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta. 2013; 1830:3956–64.
Article55. Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983; 305:712–3.
Article56. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001; 15:2137–48.57. Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004; 53:577–84.
Article58. Yau WW, Singh BK, Lesmana R, Zhou J, Sinha RA, Wong KA, et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019; 15:131–50.
Article59. Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007; 148:3080–8.
Article60. Morte B, Bernal J. Thyroid hormone action: astrocyte-neuron communication. Front Endocrinol (Lausanne). 2014; 5:82.
Article61. Barez-Lopez S, Montero-Pedrazuela A, Bosch-Garcia D, Venero C, Guadano-Ferraz A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology. 2017; 84:51–60.
Article62. Bocco BM, Werneck-de-Castro JP, Oliveira KC, Fernandes GW, Fonseca TL, Nascimento BP, et al. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-like behavior in male mice. Endocrinology. 2016; 157:3682–95.
Article63. Uter JC, Kramer UM, Schols L, Rodriguez-Fornells A, Gobel A, Heldmann M, et al. Single nucleotide polymorphisms in thyroid hormone transporter genes MCT8, MCT10 and deiodinase DIO2 contribute to inter-individual variance of executive functions and personality traits. Exp Clin Endocrinol Diabetes. 2020; 128:573–81.
Article64. Nascimento BP, Bocco BM, Fernandes GW, Fonseca TL, McAninch EA, Cardoso CV, et al. Induction of type 2 iodothyronine deiodinase after status epilepticus modifies hippocampal gene expression in male mice. Endocrinology. 2018; 159:3090–104.
Article65. Sabatino L, Federighi G, Del Seppia C, Lapi D, Costagli C, Scuri R, et al. Thyroid hormone deiodinases response in brain of spontaneausly hypertensive rats after hypotensive effects induced by mandibular extension. Endocrine. 2021; 74:100–7.
Article66. Barez-Lopez S, Grijota-Martinez C, Auso E, Fernandez-de Frutos M, Montero-Pedrazuela A, Guadano-Ferraz A. Adult mice lacking Mct8 and Dio2 proteins present alterations in peripheral thyroid hormone levels and severe brain and motor skill impairments. Thyroid. 2019; 29:1669–82.
Article67. Dora JM, Machado WE, Rheinheimer J, Crispim D, Maia AL. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur J Endocrinol. 2010; 163:427–34.
Article68. Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, et al. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002; 51:880–3.69. Nair S, Muller YL, Ortega E, Kobes S, Bogardus C, Baier LJ. Association analyses of variants in the DIO2 gene with early-onset type 2 diabetes mellitus in Pima Indians. Thyroid. 2012; 22:80–7.
Article70. Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005; 90:3472–8.
Article71. Grarup N, Andersen MK, Andreasen CH, Albrechtsen A, Borch-Johnsen K, Jorgensen T, et al. Studies of the common DIO2 Thr92Ala polymorphism and metabolic phenotypes in 7342 Danish white subjects. J Clin Endocrinol Metab. 2007; 92:363–6.72. Gumieniak O, Perlstein TS, Williams JS, Hopkins PN, Brown NJ, Raby BA, et al. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007; 49:461–6.
Article73. van der Deure WM, Peeters RP, Uitterlinden AG, Hofman A, Breteler MM, Witteman J, et al. Impact of thyroid function and polymorphisms in the type 2 deiodinase on blood pressure: the Rotterdam Study and the Rotterdam Scan Study. Clin Endocrinol (Oxf). 2009; 71:137–44.
Article74. Dhanunjaya Y, Dolia PB, Chitraa R. Type II 5’deiodinase Thr92Ala polymorphism is associated with CVD risk among type 2 diabetes mellitus patients. J Diabetes Mellitus. 2016; 6:58–68.
Article75. Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, van der Wijk HJ, et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. 2008; 17:1867–75.
Article76. Luo M, Zhou XH, Zou T, Keyim K, Dong LM. Type II deiodinase polymorphisms and serum thyroid hormone levels in patients with mild cognitive impairment. Genet Mol Res. 2015; 14:5407–16.
Article77. Hoftijzer HC, Heemstra KA, Visser TJ, le Cessie S, Peeters RP, Corssmit EP, et al. The type 2 deiodinase ORFa-Gly3Asp polymorphism (rs12885300) influences the set point of the hypothalamus-pituitary-thyroid axis in patients treated for differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2011; 96:E1527–33.
Article78. Peltsverger MY, Butler PW, Alberobello AT, Smith S, Guevara Y, Dubaz OM, et al. The -258A/G (SNP rs12885300) polymorphism of the human type 2 deiodinase gene is associated with a shift in the pattern of secretion of thyroid hormones following a TRH-induced acute rise in TSH. Eur J Endocrinol. 2012; 166:839–45.79. Sarzo B, Ballesteros V, Iniguez C, Manzano-Salgado CB, Casas M, Llop S, et al. Maternal perfluoroalkyl substances, thyroid hormones, and DIO genes: a Spanish cross-sectional study. Environ Sci Technol. 2021; 55:11144–54.80. Bunevicius A, Laws ER, Saudargiene A, Tamasauskas A, Iervasi G, Deltuva V, et al. Common genetic variations of deiodinase genes and prognosis of brain tumor patients. Endocrine. 2019; 66:563–72.
Article81. Waarsing JH, Kloppenburg M, Slagboom PE, Kroon HM, Houwing-Duistermaat JJ, Weinans H, et al. Osteoarthritis susceptibility genes influence the association between hip morphology and osteoarthritis. Arthritis Rheum. 2011; 63:1349–54.
Article82. He B, Li J, Wang G, Ju W, Lu Y, Shi Y, et al. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33:986–90.
Article83. Galecka E, Talarowska M, Orzechowska A, Gorski P, Bienkiewicz M, Szemraj J. Association of the DIO2 gene single nucleotide polymorphisms with recurrent depressive disorder. Acta Biochim Pol. 2015; 62:297–302.
Article84. Jin T, Wang L, He X, Liu M, Bai M, Rong H, et al. Association between DIO2 polymorphism and the risk of Kashin-Beck disease in the Tibetan population. J Gene Med. 2019; 21:e3123.
Article85. Zhang RQ, Zhang DD, Zhang D, Yang XL, Li Q, Wang C, et al. Crosstalk between CpG methylation and polymorphisms (CpG-SNPs) in the promotor region of DIO2 in Kashin-Beck disease. Chin Med Sci J. 2022; 37:52–9.86. Shahida B, Planck T, Asman P, Lantz M. Study of deiodinase type 2 polymorphisms in Graves’ disease and ophthalmopathy in a Swedish population. Eur Thyroid J. 2018; 7:289–93.
Article87. Leiria LB, Dora JM, Wajner SM, Estivalet AA, Crispim D, Maia AL. The rs225017 polymorphism in the 3’UTR of the human DIO2 gene is associated with increased insulin resistance. PLoS One. 2014; 9:e103960.
Article88. Guo TW, Zhang FC, Yang MS, Gao XC, Bian L, Duan SW, et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J Med Genet. 2004; 41:585–90.
Article89. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015; 11:642–52.
Article90. Wouters HJ, van Loon HC, van der Klauw MM, Elderson MF, Slagter SN, Kobold AM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017; 27:147–55.
Article91. Cantara S, Ricci C, Maino F, Marzocchi C, Pacini F, Castagna MG. Variants in MCT10 protein do not affect FT3 levels in athyreotic patients. Endocrine. 2019; 66:551–6.
Article92. Comarella AP, Vilagellin D, Bufalo NE, Euflauzino JF, de Souza Teixeira E, Miklos AB, et al. The polymorphic inheritance of DIO2 rs225014 may predict body weight variation after Graves’ disease treatment. Arch Endocrinol Metab. 2021; 64:787–95.
Article93. de Lima Beltrao FE, de Almeida Beltrao DC, Carvalhal G, de Lima Beltrao FE, de Souza Braga Filho J, de Brito Oliveira J, et al. Heterozygote advantage of the type II deiodinase Thr92Ala polymorphism on intrahospital mortality of COVID-19. J Clin Endocrinol Metab. 2022; 107:e2488–501.94. Jo S, Fonseca TL, Bocco BM, Fernandes GW, McAninch EA, Bolin AP, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest. 2019; 129:230–45.
Article95. Wang X, Chen K, Zhang C, Wang H, Li J, Wang C, et al. The type 2 deiodinase Thr92Ala polymorphism is associated with higher body mass index and fasting glucose levels: a systematic review and meta-analysis. Biomed Res Int. 2021; 2021:9914009.
Article96. Heemstra KA, Hoftijzer H, van der Deure WM, Peeters RP, Hamdy NA, Pereira A, et al. The type 2 deiodinase Thr92Ala polymorphism is associated with increased bone turnover and decreased femoral neck bone mineral density. J Bone Miner Res. 2010; 25:1385–91.
Article97. Kang YE, Kang YM, Park B, Shong M, Yi HS. Type 2 deiodinase Thr92Ala polymorphism is associated with a reduction in bone mineral density: a community-based Korean genome and epidemiology study. Clin Endocrinol (Oxf). 2020; 93:238–47.
Article98. Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009; 94:1623–9.
Article99. Castagna MG, Dentice M, Cantara S, Ambrosio R, Maino F, Porcelli T, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017; 102:1623–30.
Article100. Kerkhof HJ, Lories RJ, Meulenbelt I, Jonsdottir I, Valdes AM, Arp P, et al. A genome-wide association study identifies an osteoarthritis susceptibility locus on chromosome 7q22. Arthritis Rheum. 2010; 62:499–510.101. Evangelou E, Kerkhof HJ, Styrkarsdottir U, Ntzani EE, Bos SD, Esko T, et al. A meta-analysis of genome-wide association studies identifies novel variants associated with osteoarthritis of the hip. Ann Rheum Dis. 2014; 73:2130–6.102. Butterfield NC, Curry KF, Steinberg J, Dewhurst H, Komla-Ebri D, Mannan NS, et al. Accelerating functional gene discovery in osteoarthritis. Nat Commun. 2021; 12:467.
Article103. Yu FF, Sun L, Zhou GY, Ping ZG, Guo X, Ba Y. Meta-analysis of association studies of selenoprotein gene polymorphism and Kashin-Beck disease: an updated systematic review. Biol Trace Elem Res. 2022; 200:543–50.
Article104. McAninch EA, Rajan KB, Evans DA, Jo S, Chaker L, Peeters RP, et al. A common DIO2 polymorphism and Alzheimer disease dementia in African and European Americans. J Clin Endocrinol Metab. 2018; 103:1818–26.
Article105. E Marcondes AA, Gomez TG, Ravache TT, Batistuzzo A, Lorena FB, de Paula CS, et al. Assessment of children in the autistic spectrum disorder that carry the Thr92Ala-DIO2 polymorphism. J Endocrinol Invest. 2021; 44:1775–82.
Article106. Janowska M, Potocka N, Paszek S, Skrzypa M, Zulewicz K, Kluz M, et al. An assessment of GPX1 (rs1050450), DIO2 (rs225014) and SEPP1 (rs7579) gene polymorphisms in women with endometrial cancer. Genes (Basel). 2022; 13:188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions
- A Case of Consumptive Hypothyroidism in a 1-Month-Old Boy with Diffuse Infantile Hepatic Hemangiomas
- Glucagon: Physiological and Pharmacological Functions and Pathophysiological Significance in Type 2 Diabetes
- Association of HLA Class II and Non-HLA Gene Polymorphisms with Disease Susceptibility in Korean Children with Type 1 Diabetes Mellitus
- Strategies for Anterior Screw Fixation for Type II Odontoid Process Fracture, and Long-Term Follow-up Results