Korean J Physiol Pharmacol.
2023 May;27(3):209-220. 10.4196/kjpp.2023.27.3.209.
Edaravone alleviates lung damage in mice with hypoxic pulmonary hypertension by increasing nitric oxide synthase 3 expression
- Affiliations
-
- 1Department of Cardiovascular Medicine, The First Affiliated Hospital of Hainan Medical University, Haikou, Hainan 570102, P.R. China
- KMID: 2541633
- DOI: http://doi.org/10.4196/kjpp.2023.27.3.209
Abstract
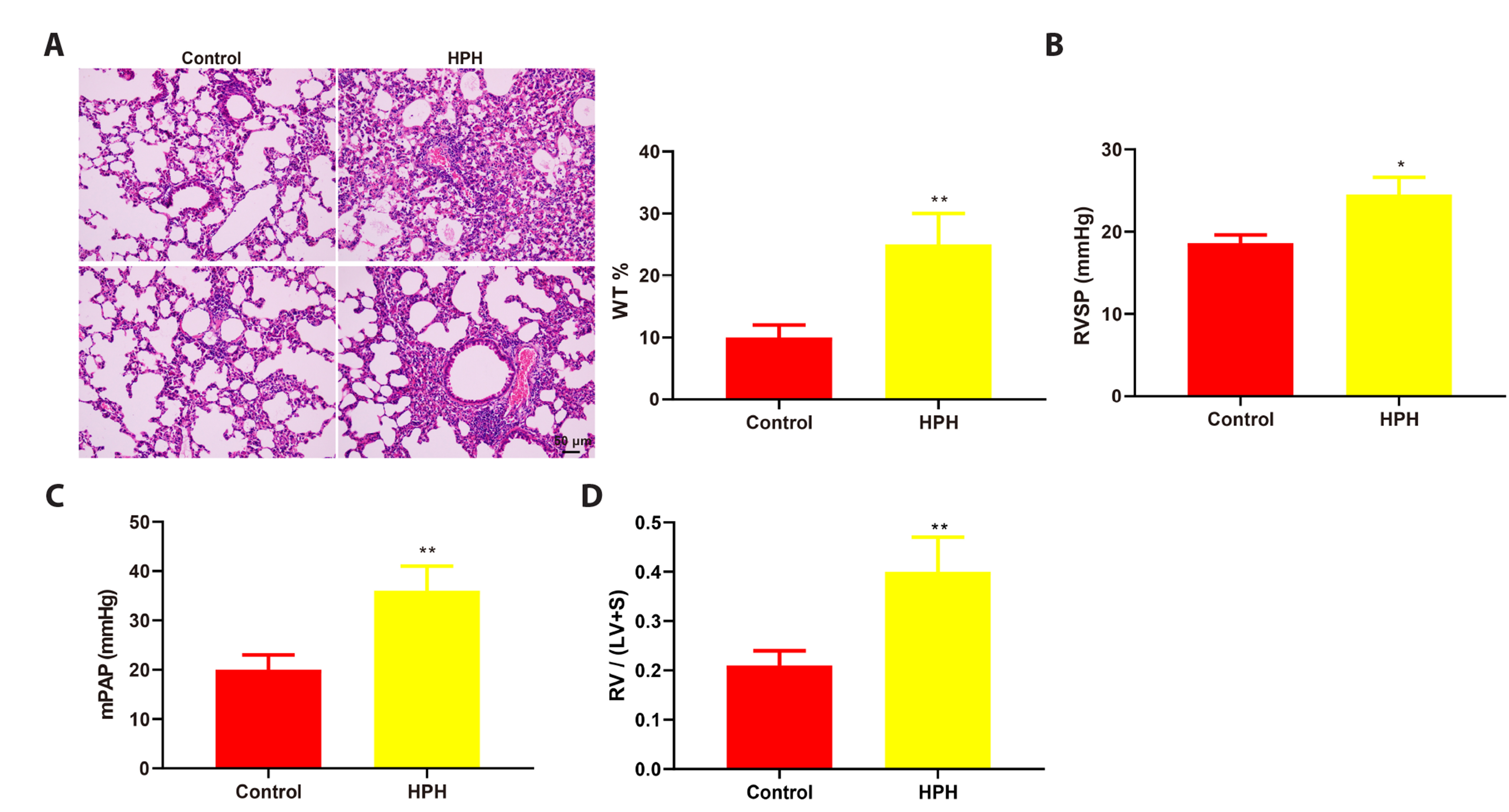
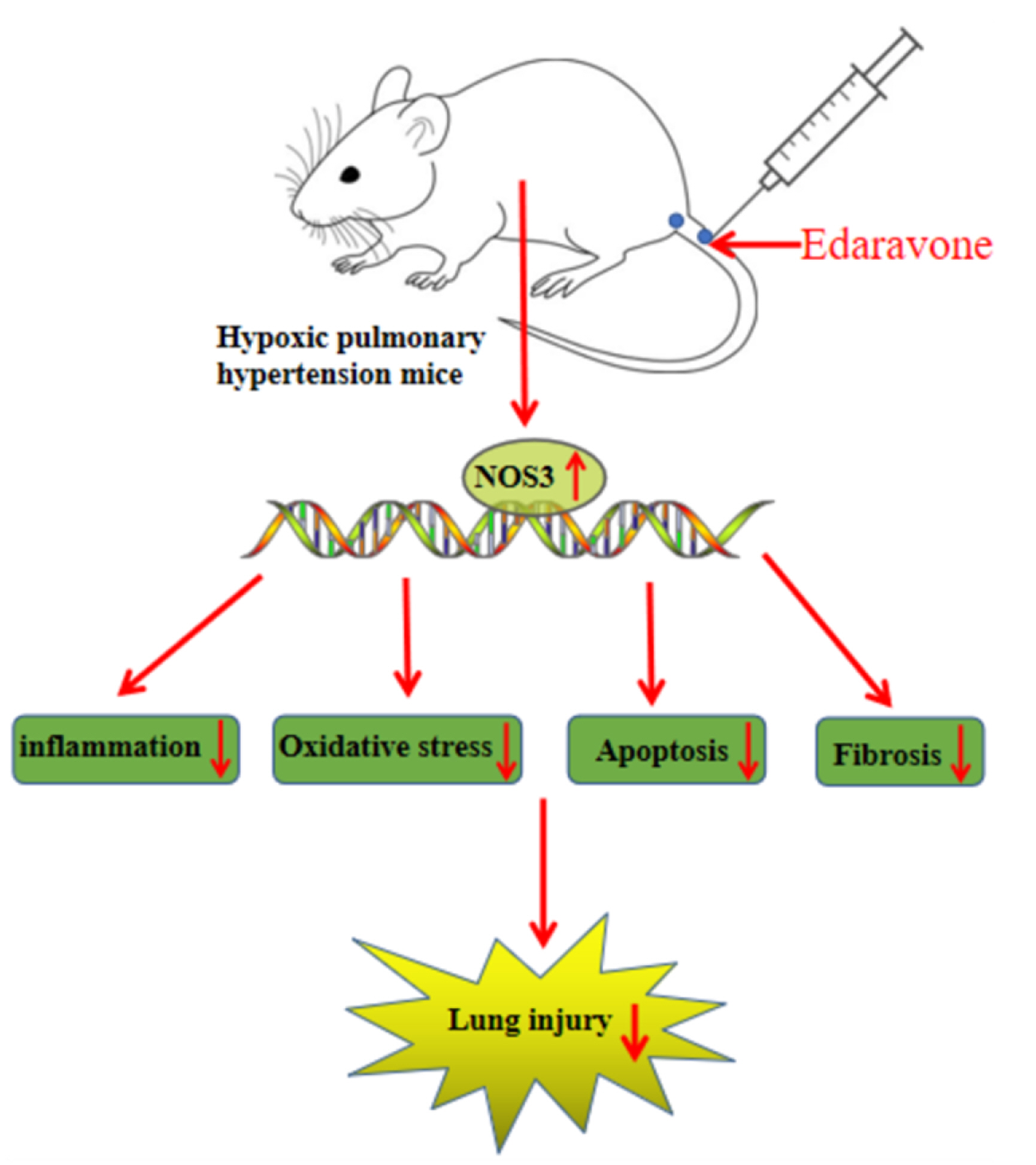
- This study is to determine the regulation of nitric oxide synthase 3 (NOS3) by edaravone in mice with hypoxic pulmonary hypertension (HPH). C57BL/6J mice were reared in a hypoxic chamber. HPH mice were treated with edaravone or edaravone + L-NMMA (a NOS inhibitor). Lung tissue was collected for histological assessment, apoptosis analysis, and detection of malondialdehyde, superoxide dismutase, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and NOS3. The levels of serum TNF-α and IL-6 were also measured. Immunohistochemistry was used to visualize the expression of α-smooth muscle actin (SMA) in pulmonary arterioles. Edaravone treatment improved hemodynamics, inhibited right ventricular hypertrophy, increased NOS3 expression, and reduced pathological changes, pulmonary artery wall thickness, apoptotic pulmonary cells, oxidative stress, and the expression of TNF-α, IL-6, and α-SMA in HPH mice. L-NMMA treatment counteracted the lung protective effects of edaravone. In conclusion, edaravone might reduce lung damage in HPH mice by increasing the expression of NOS3.
Figure
Reference
-
1. Mandras SA, Mehta HS, Vaidya A. 2020; Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc. 95:1978–1988. DOI: 10.1016/j.mayocp.2020.04.039. PMID: 32861339.
Article2. Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. 2016; A global view of pulmonary hypertension. Lancet Respir Med. 4:306–322. DOI: 10.1016/S2213-2600(15)00543-3. PMID: 26975810.
Article3. Gassmann M, Cowburn A, Gu H, Li J, Rodriguez M, Babicheva A, Jain PP, Xiong M, Gassmann NN, Yuan JX, Wilkins MR, Zhao L. 2021; Hypoxia-induced pulmonary hypertension-Utilizing experiments of nature. Br J Pharmacol. 178:121–131. DOI: 10.1111/bph.15144. PMID: 32464698.
Article4. Jaiswal MK. 2019; Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 39:733–748. DOI: 10.1002/med.21528. PMID: 30101496.
Article5. Yoshino H. 2019; Edaravone for the treatment of amyotrophic lateral sclerosis. Expert Rev Neurother. 19:185–193. DOI: 10.1080/14737175.2019.1581610. PMID: 30810406.
Article6. Tokumaru O, Shuto Y, Ogata K, Kamibayashi M, Bacal K, Takei H, Yokoi I, Kitano T. 2018; Dose-dependency of multiple free radical-scavenging activity of edaravone. J Surg Res. 228:147–153. DOI: 10.1016/j.jss.2018.03.020. PMID: 29907205.
Article7. Tajima S, Soda M, Bando M, Enomoto M, Yamasawa H, Ohno S, Takada T, Suzuki E, Gejyo F, Sugiyama Y. 2008; Preventive effects of edaravone, a free radical scavenger, on lipopolysaccharide-induced lung injury in mice. Respirology. 13:646–653. DOI: 10.1111/j.1440-1843.2008.01322.x. PMID: 18713088.
Article8. Yamaguchi S, Hussein MH, Daoud GA, Goto T, Kato S, Kakita H, Mizuno H, Ito T, Fukuda S, Kato I, Suzuki S, Hashimoto T, Togari H. 2011; Edaravone, a hydroxyl radical scavenger, ameliorates the severity of pulmonary hypertension in a porcine model of neonatal sepsis. Tohoku J Exp Med. 223:235–241. DOI: 10.1620/tjem.223.235. PMID: 21415574.
Article9. Król M, Kepinska M. 2020; Human nitric oxide synthase-its functions, polymorphisms, and inhibitors in the context of inflammation, diabetes and cardiovascular diseases. Int J Mol Sci. 22:56. DOI: 10.3390/ijms22010056. PMID: 33374571. PMCID: PMC7793075. PMID: f05930ff4d56445f82af89cee35fe38d.
Article10. Fish JE, Marsden PA. 2006; Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci. 63:144–162. DOI: 10.1007/s00018-005-5421-8. PMID: 16416260.
Article11. Tejero J, Shiva S, Gladwin MT. 2019; Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev. 99:311–379. DOI: 10.1152/physrev.00036.2017. PMID: 30379623. PMCID: PMC6442925.
Article12. Lázár Z, Mészáros M, Bikov A. 2020; The nitric oxide pathway in pulmonary arterial hypertension: pathomechanism, biomarkers and drug targets. Curr Med Chem. 27:7168–7188. DOI: 10.2174/0929867327666200522215047. PMID: 32442078.
Article13. Chalupsky K, Kračun D, Kanchev I, Bertram K, Görlach A. 2015; Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. 23:1076–1091. DOI: 10.1089/ars.2015.6329. PMID: 26414244. PMCID: PMC4657514.
Article14. Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. 2000; Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 105:1555–1562. DOI: 10.1172/JCI8678. PMID: 10841514. PMCID: PMC300850.
Article15. Dias MB, Almeida MC, Carnio EC, Branco LG. 2005; Role of nitric oxide in tolerance to lipopolysaccharide in mice. J Appl Physiol (1985). 98:1322–1327. DOI: 10.1152/japplphysiol.01243.2004. PMID: 15579566.
Article16. Soejima M, Koda Y. 2008; TaqMan-based real-time PCR for genotyping common polymorphisms of haptoglobin (HP1 and HP2). Clin Chem. 54:1908–1913. DOI: 10.1373/clinchem.2008.113126. PMID: 18787013.
Article17. Hoeper MM, Ghofrani HA, Grünig E, Klose H, Olschewski H, Rosenkranz S. 2017; Pulmonary hypertension. Dtsch Arztebl Int. 114:73–84. DOI: 10.3238/arztebl.2016.0073. PMID: 28241922. PMCID: PMC5331483.
Article18. Hu CJ, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, McKeon B, Mouradian G, Li M, Riddle S, Pugliese SC, Brown RD, Wallace EM, Graham BB, Frid MG, Stenmark KR. 2019; Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J. 54:1900378. DOI: 10.1183/13993003.00378-2019. PMID: 31515405. PMCID: PMC6911916.
Article19. Ahmadinejad F, Geir Møller S, Hashemzadeh-Chaleshtori M, Bidkhori G, Jami MS. 2017; Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants (Basel). 6:51. DOI: 10.3390/antiox6030051. PMID: 28698499. PMCID: PMC5618079. PMID: c8a47e0f322744768b4b58b9559af7cd.
Article20. Bao Q, Hu P, Xu Y, Cheng T, Wei C, Pan L, Shi J. 2018; Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 12:6794–6805. DOI: 10.1021/acsnano.8b01994. PMID: 29932327.
Article21. Ishii H, Petrenko AB, Sasaki M, Satoh Y, Kamiya Y, Tobita T, Furutani K, Matsuhashi M, Kohno T, Baba H. 2018; Free radical scavenger edaravone produces robust neuroprotection in a rat model of spinal cord injury. Brain Res. 1682:24–35. DOI: 10.1016/j.brainres.2017.12.035. PMID: 29294349.
Article22. Koike N, Sasaki A, Murakami T, Suzuki K. 2021; Effect of edaravone against cisplatin-induced chronic renal injury. Drug Chem Toxicol. 44:437–446. DOI: 10.1080/01480545.2019.1604740. PMID: 31064223.
Article23. Masuda T, Shimazawa M, Hara H. 2017; Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (edaravone). Oxid Med Cell Longev. 2017:9208489. DOI: 10.1155/2017/9208489. PMID: 28194256. PMCID: PMC5286467. PMID: 58e49f5a8578417c833fad724be80ff7.
Article24. Song Y, Bei Y, Xiao Y, Tong HD, Wu XQ, Chen MT. 2018; Edaravone, a free radical scavenger, protects neuronal cells' mitochondria from ischemia by inactivating another new critical factor of the 5-lipoxygenase pathway affecting the arachidonic acid metabolism. Brain Res. 1690:96–104. DOI: 10.1016/j.brainres.2018.03.006. PMID: 29551652.
Article25. Kassab AA, Aboregela AM, Shalaby AM. 2020; Edaravone attenuates lung injury in a hind limb ischemia-reperfusion rat model: a histological, immunohistochemical and biochemical study. Ann Anat. 228:151433. DOI: 10.1016/j.aanat.2019.151433. PMID: 31678401.
Article26. Han CH, Guan ZB, Zhang PX, Fang HL, Li L, Zhang HM, Zhou FJ, Mao YF, Liu WW. 2018; Oxidative stress induced necroptosis activation is involved in the pathogenesis of hyperoxic acute lung injury. Biochem Biophys Res Commun. 495:2178–2183. DOI: 10.1016/j.bbrc.2017.12.100. PMID: 29269294.
Article27. Guo H, Yang R, He J, Chen K, Yang W, Liu J, Xiao K, Li H. 2021; Edaravone combined with dexamethasone exhibits synergic effects on attenuating smoke-induced inhalation lung injury in rats. Biomed Pharmacother. 141:111894. DOI: 10.1016/j.biopha.2021.111894. PMID: 34225014.
Article28. Xiao C, Du M, Liu Y, Yu Y, Yang J. 2021; Edaravone attenuates smoke inhalation injury in rats by the Notch pathway. Am J Transl Res. 13:4712–4718. PMID: 34150051. PMCID: PMC8205801.29. Xiao C, Yu Y, Liu Y, Yang J. 2021; Aerosol inhalation of edaravone can improve inflammation, oxidative stress and pulmonary function of rats with smoke inhalation injury by down-regulating miR-320. Am J Transl Res. 13:2563–2570. PMID: 34017415. PMCID: PMC8129403.30. Wang X, Lai R, Su X, Chen G, Liang Z. 2018; Edaravone attenuates lipopolysaccharide-induced acute respiratory distress syndrome associated early pulmonary fibrosis via amelioration of oxidative stress and transforming growth factor-β1/Smad3 signaling. Biochem Biophys Res Commun. 495:706–712. DOI: 10.1016/j.bbrc.2017.10.165. PMID: 29102631.
Article31. Pan Y, Li W, Feng Y, Xu J, Cao H. 2020; Edaravone attenuates experimental asthma in mice through induction of HO-1 and the Keap1/Nrf2 pathway. Exp Ther Med. 19:1407–1416. DOI: 10.3892/etm.2019.8351.
Article32. Cotta Filho CK, Oliveira-Paula GH, Rondon Pereira VC, Lacchini R. 2020; Clinically relevant endothelial nitric oxide synthase polymorphisms and their impact on drug response. Expert Opin Drug Metab Toxicol. 16:927–951. DOI: 10.1080/17425255.2020.1804857. PMID: 32746648.
Article33. Abbasi H, Dastgheib SA, Hadadan A, Karimi-Zarchi M, Javaheri A, Meibodi B, Zanbagh L, Tabatabaei RS, Neamatzadeh H. 2021; Association of endothelial nitric oxide synthase 894G > T polymorphism with preeclampsia risk: a systematic review and meta-analysis based on 35 studies. Fetal Pediatr Pathol. 40:455–470. DOI: 10.1080/15513815.2019.1710880. PMID: 31920131.
Article34. Chen XJ, Qiu CG, Kong XD, Ren SM, Dong JZ, Gu HP, Chen YW, Tao HL, Sarbesh J. 2018; The association between an endothelial nitric oxide synthase gene polymorphism and coronary heart disease in young people and the underlying mechanism. Mol Med Rep. 17:3928–3934. DOI: 10.3892/mmr.2017.8314. PMID: 29359785.
Article35. Dong H, Wang ZH, Dong B, Hu YN, Zhao HY. 2018; Endothelial nitric oxide synthase (-786T>C) polymorphism and migraine susceptibility: a meta-analysis. Medicine (Baltimore). 97:e12241. DOI: 10.1097/MD.0000000000012241. PMID: 30200152. PMCID: PMC6133471.36. Paschoal EHA, Yamaki VN, Teixeira RKC, Paschoal Junior FM, Jong-A-Liem GS, Teixeira MJ, Yamada ES, Ribeiro-Dos-Santos Â, Bor-Seng-Shu E. 2018; Relationship between endothelial nitric oxide synthase (eNOS) and natural history of intracranial aneurysms: meta-analysis. Neurosurg Rev. 41:87–94. DOI: 10.1007/s10143-016-0761-4. PMID: 27339197.
Article37. Saluja A, Saraswathy KN, Thakur S, Margekar S, Goyal A, Dhamija RK. 2021; Endothelial nitric oxide synthase (Glu298Asp) polymorphism is associated significantly with ischemic stroke presenting with seizures and altered sensorium. Neurol India. 69:686–691. DOI: 10.4103/0028-3886.319217. PMID: 34169869.
Article38. Drucker NA, Jensen AR, Te Winkel JP, Ferkowicz MJ, Markel TA. 2018; Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J Pediatr Surg. 53:1208–1214. DOI: 10.1016/j.jpedsurg.2018.02.087. PMID: 29618412. PMCID: PMC5994357.
Article39. Gao W, Jiang T, Liu YH, Ding WG, Guo CC, Cui XG. 2019; Endothelial progenitor cells attenuate the lung ischemia/reperfusion injury following lung transplantation via the endothelial nitric oxide synthase pathway. J Thorac Cardiovasc Surg. 157:803–814. DOI: 10.1016/j.jtcvs.2018.08.092. PMID: 30391008.
Article40. Zhu ML, Gao ZT, Lu JX, Wang Y, Wang G, Zhu TT, Li P, Liu C, Wang SX, Yang L. 2020; Amorphous nano-selenium quantum dots prevent pulmonary arterial hypertension through recoupling endothelial nitric oxide synthase. Aging (Albany NY). 13:3368–3385. DOI: 10.18632/aging.202215. PMID: 33323558. PMCID: PMC7906187.
Article41. Ogoshi T, Tsutsui M, Kido T, Sakanashi M, Naito K, Oda K, Ishimoto H, Yamada S, Wang KY, Toyohira Y, Izumi H, Masuzaki H, Shimokawa H, Yanagihara N, Yatera K, Mukae H. 2018; Protective role of myelocytic nitric oxide synthases against hypoxic pulmonary hypertension in mice. Am J Respir Crit Care Med. 198:232–244. DOI: 10.1164/rccm.201709-1783OC. PMID: 29480750.
Article42. Jin Q, Su H, Yang R, Tan Y, Li B, Yi W, Dong Q, Zhang H, Xing W, Sun X. 2021; C1q/TNF-related protein-9 ameliorates hypoxia-induced pulmonary hypertension by regulating secretion of endothelin-1 and nitric oxide mediated by AMPK in rats. Sci Rep. 11:11372. DOI: 10.1038/s41598-021-90779-2. PMID: 34059748. PMCID: PMC8166879. PMID: 95aa1ae4c90e4da9a8ceb92cb5671961.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- Alteration of Nitric Oxide Synthase Subtype Expression in Contralateral Testis of Rat in Response to Unilateral Testicular Torsion Followed by Detorsion
- Expression profile of mitochondrial voltage-dependent anion channel-1 (VDAC1) influenced genes is associated with pulmonary hypertension
- Immunohistochemical Expression of Placental Nitric Oxide Synthase in Preeclampsia and Normal Pregnancy