Korean Circ J.
2023 Mar;53(3):151-167. 10.4070/kcj.2022.0242.
LncRNA AC005332.7 Inhibited Ferroptosis to Alleviate Acute Myocardial Infarction Through Regulating miR-331-3p/CCND2 Axis
- Affiliations
-
- 1Department of Cardiology, The Affiliated Hospital of Guilin Medical University, Guilin, P.R. China
- KMID: 2540393
- DOI: http://doi.org/10.4070/kcj.2022.0242
Abstract
- Background and Objectives
Acute myocardial infarction (AMI) often occurs suddenly and leads to fatal consequences. Ferroptosis is closely related to the progression of AMI. However, the specific mechanism of ferroptosis in AMI remains unclear.
Methods
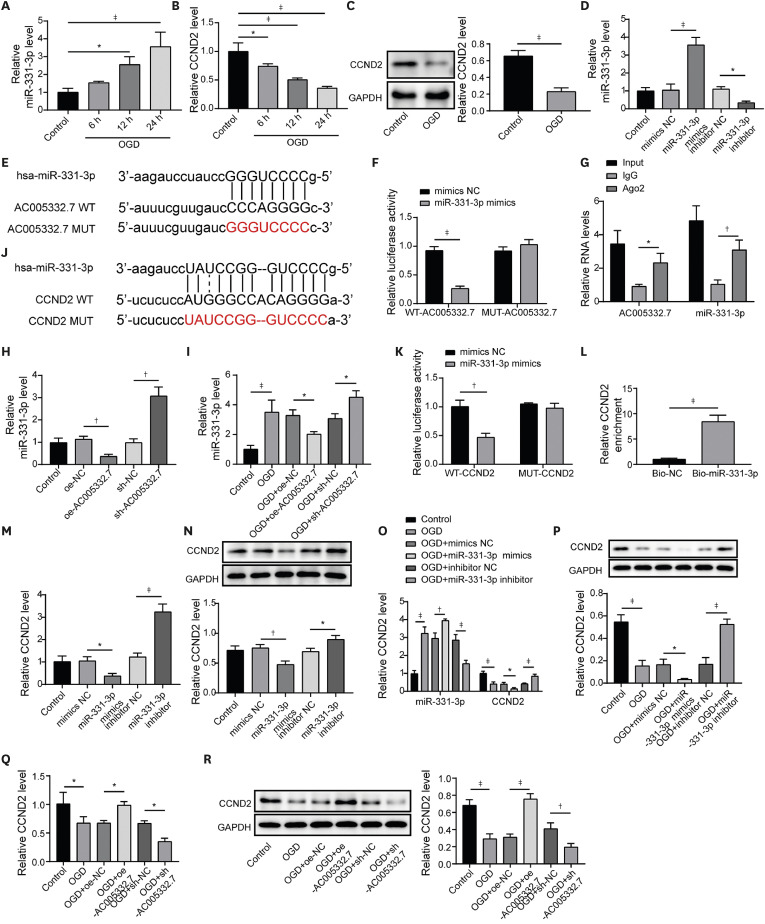
We constructed a cell model of AMI using AC16 cells under oxygen and glucose deprivation (OGD) conditions and a mice model of AMI using the left anterior descending (LAD) ligation. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide was employed to determine cell viability. The levels of lactate dehydrogenase, creatine kinase, reactive oxygen species (ROS), glutathione (GSH), malondialdehyde (MDA), and iron were measured using corresponding kits. Dual luciferase reporter gene assay, RNAbinding protein immunoprecipitation, and RNA pull-down were performed to validate the correlations among AC005332.7, miR-331-3p, and cyclin D2 (CCND2). Hematoxylin and eosin staining was employed to evaluate myocardial damage.
Results
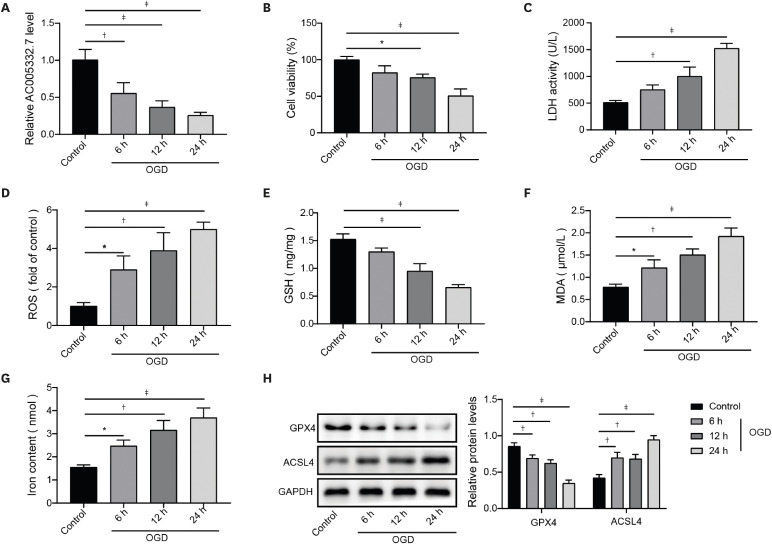
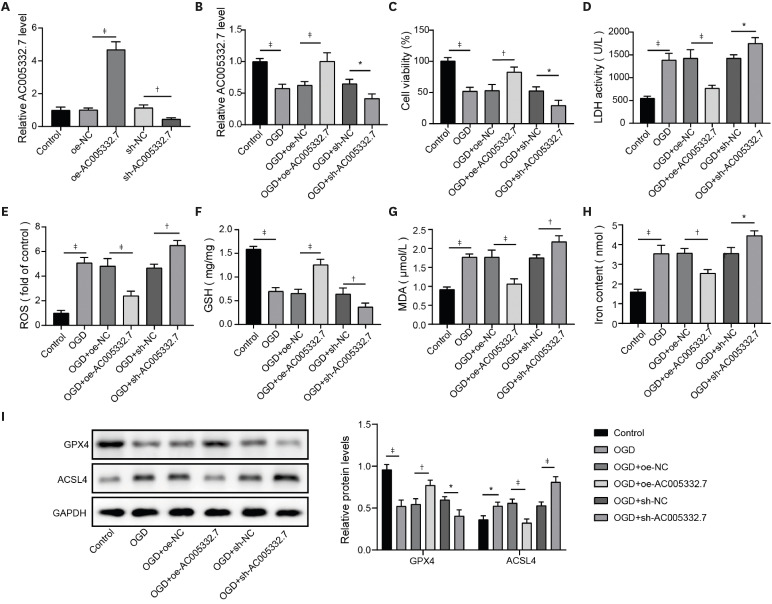
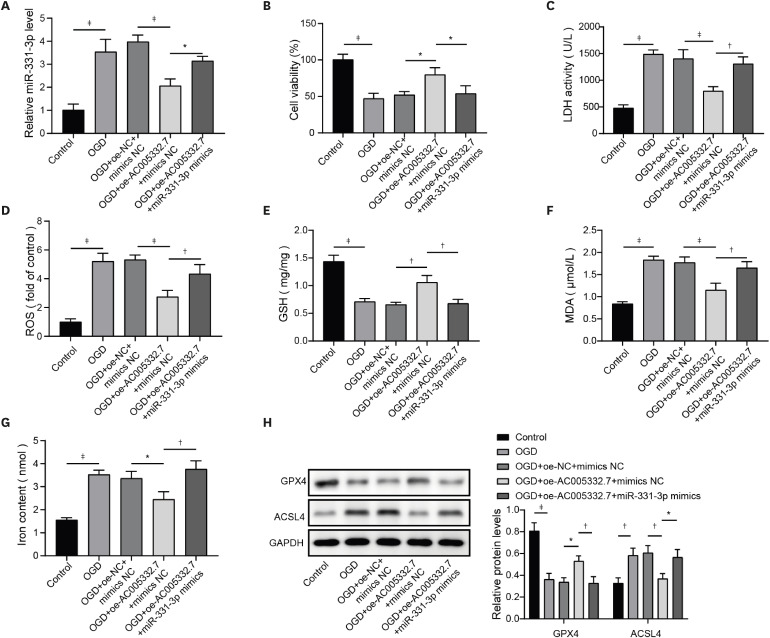
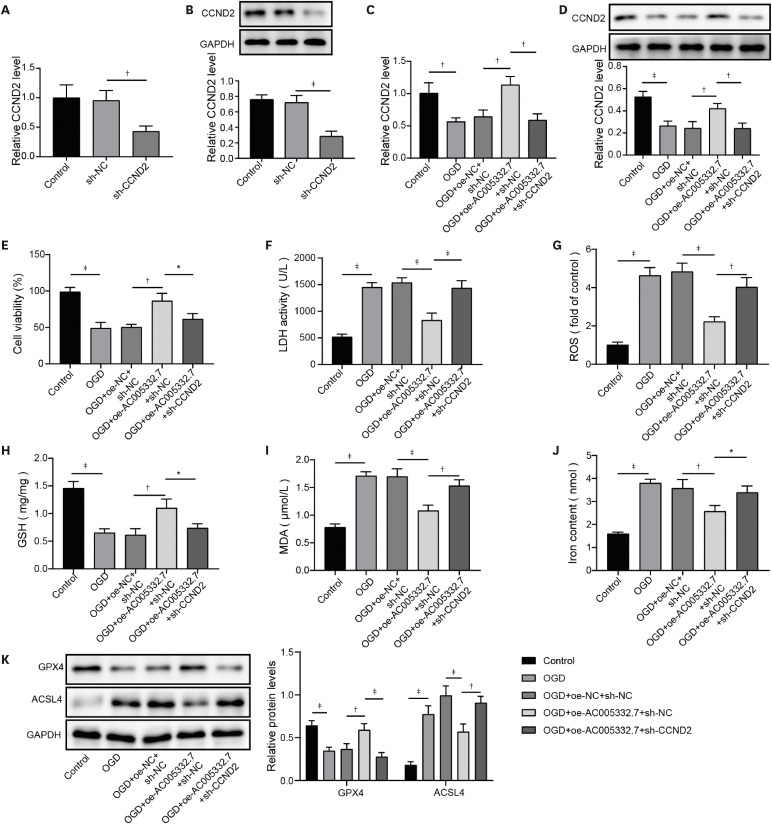
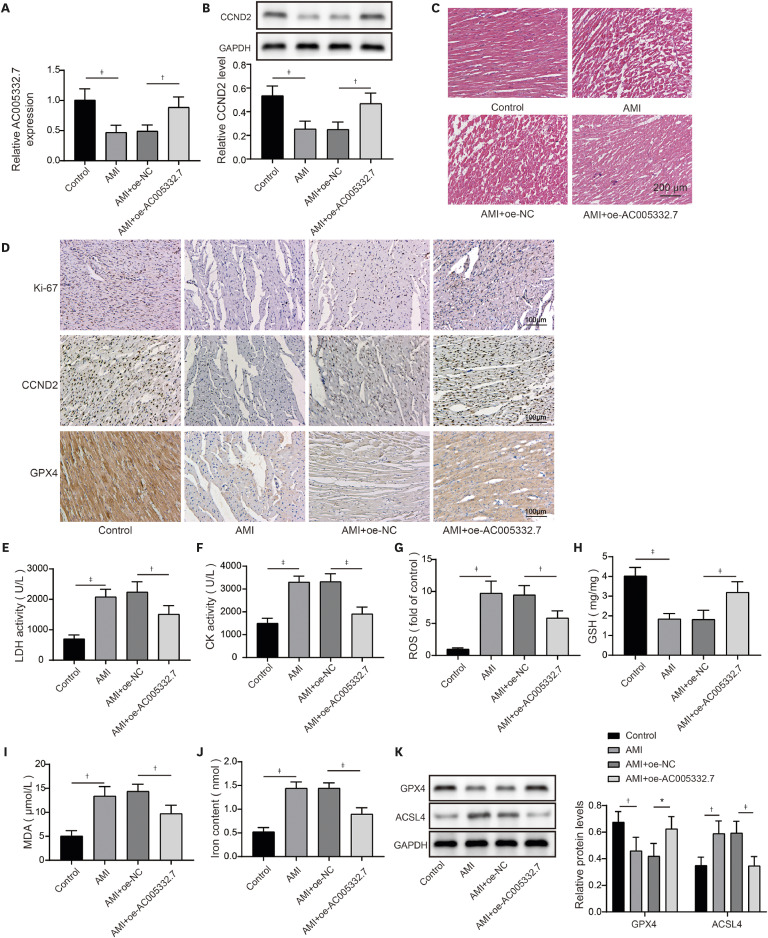
AC005332.7 and CCND2 were lowly expressed, while miR-331-3p was highly expressed in vivo and in vitro models of AMI. AC005332.7 sufficiency reduced ROS, MDA, iron, and ACSL4 while boosting the GSH and GPX4, indicating that AC005332.7 sufficiency impeded ferroptosis to improve cardiomyocyte injury in AMI. Mechanistically, AC005332.7 interacted with miR-331-3p, and miR-331-3p targeted CCND2. Additionally, miR-331-3p overexpression or CCND2 depletion abolished the suppressive impact of AC005332.7 on ferroptosis in OGD-induced AC16 cells. Moreover, AC005332.7 overexpression suppressed ferroptosis in mice models of AMI.
Conclusions
AC005332.7 suppressed ferroptosis in OGD-induced AC16 cells and LAD ligation-operated mice through modulating miR-331-3p/CCND2 axis, thereby mitigating the cardiomyocyte injury in AMI, which proposed novel targets for AMI treatment.
Keyword
Figure
Reference
-
1. Widimsky P, Coram R, Abou-Chebl A. Reperfusion therapy of acute ischaemic stroke and acute myocardial infarction: similarities and differences. Eur Heart J. 2014; 35:147–155. PMID: 24096325.2. Sharma V, Bell RM, Yellon DM. Targeting reperfusion injury in acute myocardial infarction: a review of reperfusion injury pharmacotherapy. Expert Opin Pharmacother. 2012; 13:1153–1175. PMID: 22594845.3. Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016; 26:165–176. PMID: 26653790.4. Fan K, Huang W, Qi H, et al. The Egr-1/miR-15a-5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur J Pharmacol. 2021; 909:174403. PMID: 34339707.5. Zhang M, Zhang Z, Hu J, Zhou S, Ai W. Knockdown of long noncoding RNA MIAT attenuates hypoxia-induced cardiomyocyte injury by regulating the miR-488-3p/Wnt/β-catenin pathway. Cell Biol Int. 2023; 47:63–74. PMID: 36273414.6. Chen ML, Hong CG, Yue T, et al. Inhibition of miR-331-3p and miR-9-5p ameliorates Alzheimer’s disease by enhancing autophagy. Theranostics. 2021; 11:2395–2409. PMID: 33500732.7. Horváth M, Horváthová V, Hájek P, et al. MicroRNA-331 and microRNA-151-3p as biomarkers in patients with ST-segment elevation myocardial infarction. Sci Rep. 2020; 10:5845. PMID: 32246100.8. Song X, Shan D, Chen J, Jing Q. miRNAs and lncRNAs in vascular injury and remodeling. Sci China Life Sci. 2014; 57:826–835. PMID: 25104456.9. Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018; 22:5768–5775. PMID: 30188595.10. Toischer K, Zhu W, Hünlich M, et al. Cardiomyocyte proliferation prevents failure in pressure overload but not volume overload. J Clin Invest. 2017; 127:4285–4296. PMID: 29083322.11. Zhu W, Zhao M, Mattapally S, Chen S, Zhang J. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes: remuscularization of injured ventricle. Circ Res. 2018; 122:88–96. PMID: 29018036.12. Ni T, Huang X, Pan S, Lu Z. Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. J Cell Mol Med. 2021; 25:9995–10007. PMID: 34609043.13. Bai WW, Tang ZY, Shan TC, et al. Up-regulation of paired-related homeobox 2 promotes cardiac fibrosis in mice following myocardial infarction by targeting of Wnt5a. J Cell Mol Med. 2020; 24:2319–2329. PMID: 31880857.14. Zhou M, Zou YG, Xue YZ, et al. Long non-coding RNA H19 protects acute myocardial infarction through activating autophagy in mice. Eur Rev Med Pharmacol Sci. 2018; 22:5647–5651. PMID: 30229841.15. Zheng HF, Sun J, Zou ZY, Zhang Y, Hou GY. MiRNA-488-3p suppresses acute myocardial infarction-induced cardiomyocyte apoptosis via targeting ZNF791. Eur Rev Med Pharmacol Sci. 2019; 23:4932–4939. PMID: 31210328.16. Castro-Dominguez Y, Dharmarajan K, McNamara RL. Predicting death after acute myocardial infarction. Trends Cardiovasc Med. 2018; 28:102–109. PMID: 28826668.17. Song Y, Wang B, Zhu X, et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021; 37:51–64. PMID: 32535745.18. Yang J, Huang X, Hu F, Fu X, Jiang Z, Chen K. LncRNA ANRIL knockdown relieves myocardial cell apoptosis in acute myocardial infarction by regulating IL-33/ST2. Cell Cycle. 2019; 18:3393–3403. PMID: 31674275.19. Hu H, Wu J, Li D, Zhou J, Yu H, Ma L. Knockdown of lncRNA MALAT1 attenuates acute myocardial infarction through miR-320-Pten axis. Biomed Pharmacother. 2018; 106:738–746. PMID: 29990866.20. Mao C, Wang X, Liu Y, et al. A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018; 78:3484–3496. PMID: 29588351.21. Wei XB, Jiang WQ, Zeng JH, et al. Exosome-derived lncRNA NEAT1 exacerbates sepsis-associated encephalopathy by promoting ferroptosis through regulating miR-9-5p/TFRC and GOT1 axis. Mol Neurobiol. 2022; 59:1954–1969. PMID: 35038133.22. Zhang JK, Zhang Z, Guo ZA, et al. The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis. 2022; 32:515–527. PMID: 34953631.23. Hao X, Wei H. LncRNA H19 alleviates sepsis-induced acute lung injury by regulating the miR-107/TGFBR3 axis. BMC Pulm Med. 2022; 22:371. PMID: 36180862.24. Song X, Chen Y, Peng Y, et al. LncRNA-PAX8-AS1 silencing decreases cell viability, enhances apoptosis, and suppresses doxorubicin resistance in myeloid leukemia via the miR-378g/ERBB2 axis. Evid Based Complement Alternat Med. 2022; 2022:2295044. PMID: 36248434.25. Wang S, Liu Y, Hu X, et al. Identification of ceRNA (lncRNA-miRNA-mRNA) regulatory network in myocardial fibrosis after acute myocardial infarction. Int J Gen Med. 2021; 14:9977–9990. PMID: 34984022.26. Zhou J, He S, Wang B, et al. Construction and bioinformatics analysis of circRNA-miRNA-mRNA network in acute myocardial infarction. Front Genet. 2022; 13:854993. PMID: 35422846.27. Gong S, Ying L, Fan Y, Sun Z. Fentanyl inhibits lung cancer viability and invasion via upregulation of miR-331-3p and repression of HDAC5. Onco Targets Ther. 2020; 13:13131–13141. PMID: 33380803.28. Zhao M, Nakada Y, Wei Y, et al. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation. 2021; 144:210–228. PMID: 33951921.29. Hu Y, Jin G, Li B, et al. Suppression of miRNA let-7i-5p promotes cardiomyocyte proliferation and repairs heart function post injury by targetting CCND2 and E2F2. Clin Sci (Lond). 2019; 133:425–441. PMID: 30679264.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p
- DNM3OS Facilitates Ovarian Cancer Progression by Regulating miR-193a-3p/MAP3K3 Axis
- LncRNA XIST promotes carboplatin resistance of ovarian cancer through activating autophagy via targeting miR-506-3p/FOXP1 axis
- Long Noncoding RNA PVT1 Promotes Stemness and Temozolomide Resistance through miR-365/ELF4/SOX2 Axis in Glioma
- LINC00662 Promotes Oral Squamous Cell Carcinoma Cell Growth and Metastasis through miR-144-3p/EZH2 Axis