Korean J Physiol Pharmacol.
2023 Mar;27(2):177-185. 10.4196/kjpp.2023.27.2.177.
KLF9 deficiency protects the heart from inflammatory injury triggered by myocardial infarction
- Affiliations
-
- 1Department of Cardiology, Heji Hospital of Changzhi Medical College, Changzhi 046011, China
- KMID: 2539572
- DOI: http://doi.org/10.4196/kjpp.2023.27.2.177
Abstract
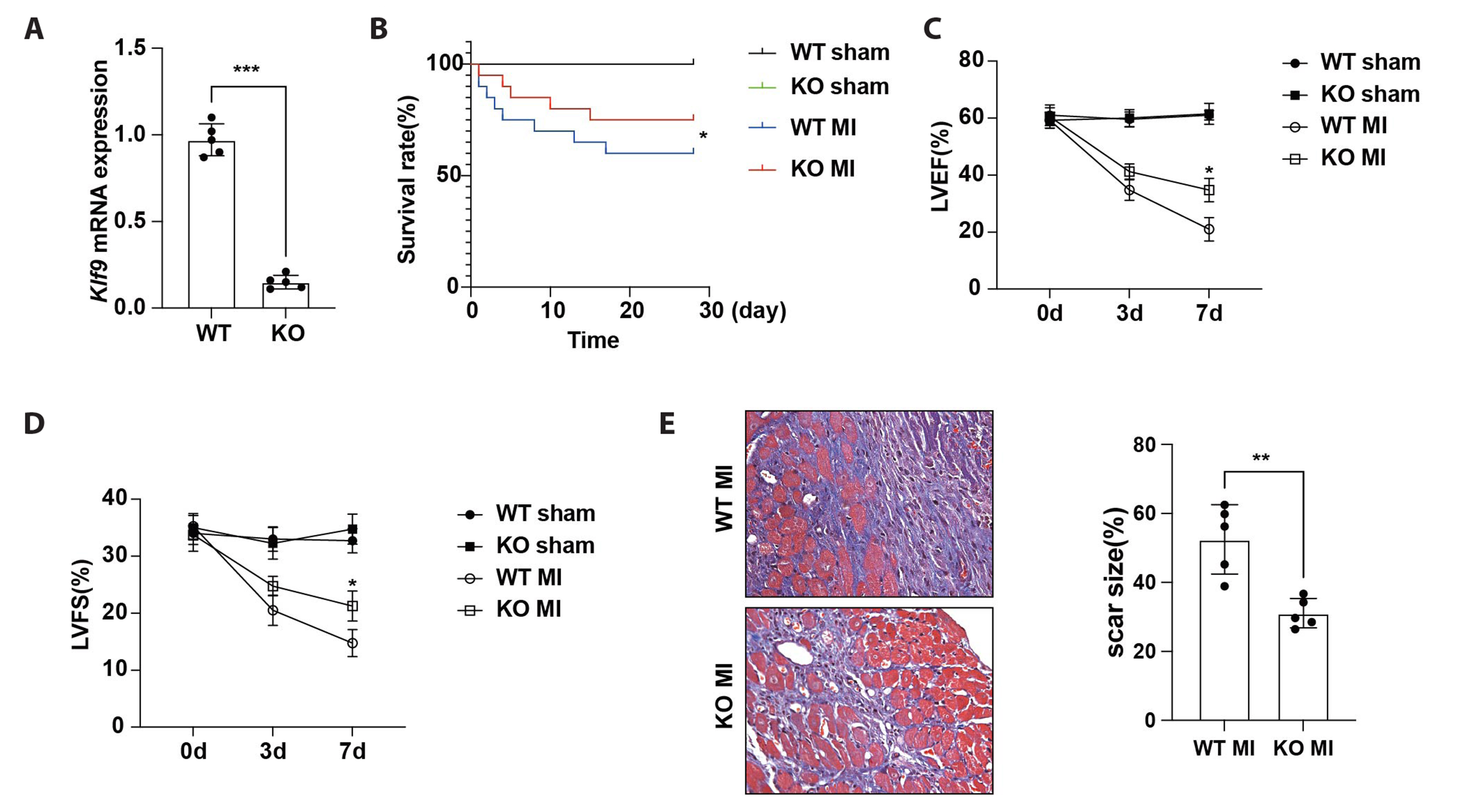
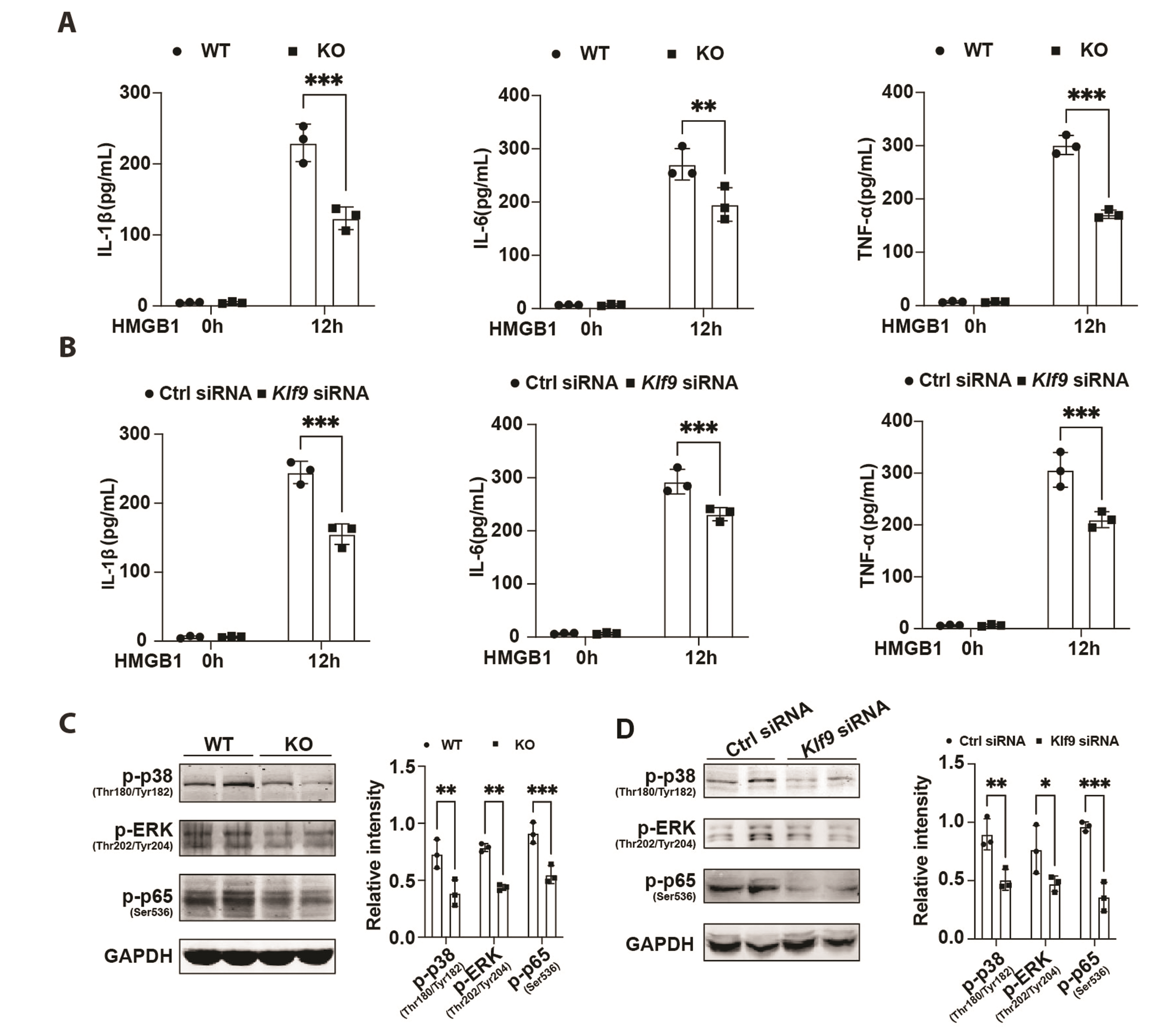
- The excessive inflammatory response induced by myocardial infarction exacerbates heart injury and leads to the development of heart failure. Recent studies have confirmed the involvement of multiple transcription factors in the modulation of cardiovascular disease processes. However, the role of KLF9 in the inflammatory response induced by cardiovascular diseases including myocardial infarction remains unclear. Here, we found that the expression of KLF9 significantly increased during myocardial infarction. Besides, we also detected high expression of KLF9 in infiltrated macrophages after myocardial infarction. Our functional studies revealed that KLF9 deficiency prevented cardiac function and adverse cardiac remodeling. Furthermore, the downregulation of KLF9 inhibited the activation of NF-κB and MAPK signaling, leading to the suppression of inflammatory responses of macrophages triggered by myocardial infarction. Mechanistically, KLF9 was directly bound to the TLR2 promoter to enhance its expression, subsequently promoting the activation of inflammation-related signaling pathways. Our results suggested that KLF9 is a pro-inflammatory transcription factor in macrophages and targeting KLF9 may be a novel therapeutic strategy for ischemic heart disease.
Figure
Reference
-
1. Jung SE, Kim SW, Jeong S, Moon H, Choi WS, Lim S, Lee S, Hwang KC, Choi JW. 2021; MicroRNA-26a/b-5p promotes myocardial infarction-induced cell death by downregulating cytochrome c oxidase 5a. Exp Mol Med. 53:1332–1343. DOI: 10.1038/s12276-021-00665-0. PMID: 34518647. PMCID: PMC8492744. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114853781&origin=inward.
Article2. Daseke MJ 2nd, Tenkorang MAA, Chalise U, Konfrst SR, Lindsey ML. 2020; Cardiac fibroblast activation during myocardial infarction wound healing: fibroblast polarization after MI. Matrix Biol. 91-92:109–116. DOI: 10.1016/j.matbio.2020.03.010. PMID: 32446909. PMCID: PMC7434699. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85086153463&origin=inward.
Article3. Zeigler AC, Nelson AR, Chandrabhatla AS, Brazhkina O, Holmes JW, Saucerman JJ. 2020; Computational model predicts paracrine and intracellular drivers of fibroblast phenotype after myocardial infarction. Matrix Biol. 91-92:136–151. DOI: 10.1016/j.matbio.2020.03.007. PMID: 32209358. PMCID: PMC7434705. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085143848&origin=inward.
Article4. Yerra VG, Advani A. 2022; Role of CCR2-positive macrophages in pathological ventricular remodelling. Biomedicines. 10:661. DOI: 10.3390/biomedicines10030661. PMID: 35327464. PMCID: PMC8945438. PMID: f733589450fe47079343e0fd631a88d8. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85127011926&origin=inward.
Article5. Lambert JM, Lopez EF, Lindsey ML. 2008; Macrophage roles following myocardial infarction. Int J Cardiol. 130:147–158. DOI: 10.1016/j.ijcard.2008.04.059. PMID: 18656272. PMCID: PMC2857604. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=54949106928&origin=inward.
Article6. Silvis MJM, Kaffka Genaamd Dengler SE, Odille CA, Mishra M, van der Kaaij NP, Doevendans PA, Sluijter JPG, de Kleijn DPV, de Jager SCA, Bosch L, van Hout GPJ. 2020; Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol. 11:599511. DOI: 10.3389/fimmu.2020.599511. PMID: 33363540. PMCID: PMC7752942. PMID: 2ad6034569004103aea0b56035843858. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098089661&origin=inward.
Article7. Vetreno RP, Qin L, Coleman LG Jr, Crews FT. 2021; Increased toll-like receptor-MyD88-NFκB-proinflammatory neuroimmune signaling in the orbitofrontal cortex of humans with alcohol use disorder. Alcohol Clin Exp Res. 45:1747–1761. DOI: 10.1111/acer.14669. PMID: 34415075. PMCID: PMC8526379. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85113168939&origin=inward.
Article8. Lu J, Yue Y, Xiong S. 2021; Extracellular HMGB1 augments macrophage inflammation by facilitating the endosomal accumulation of ALD-DNA via TLR2/4-mediated endocytosis. Biochim Biophys Acta Mol Basis Dis. 1867:166184. DOI: 10.1016/j.bbadis.2021.166184. PMID: 34087422. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107562801&origin=inward.
Article9. van Hout GP, Arslan F, Pasterkamp G, Hoefer IE. 2016; Targeting danger-associated molecular patterns after myocardial infarction. Expert Opin Ther Targets. 20:223–239. DOI: 10.1517/14728222.2016.1088005. PMID: 26420647. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84957952298&origin=inward.
Article10. Tiller C, Reindl M, Holzknecht M, Lechner I, Simma F, Schwaiger J, Mayr A, Klug G, Bauer A, Reinstadler SJ, Metzler B. 2021; High sensitivity C-reactive protein is associated with worse infarct healing after revascularized ST-elevation myocardial infarction. Int J Cardiol. 328:191–196. DOI: 10.1016/j.ijcard.2020.12.006. PMID: 33309637. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85097799180&origin=inward.
Article11. Chhunchha B, Kubo E, Singh DP. 2022; Switching of redox signaling by Prdx6 expression decides cellular fate by hormetic phenomena involving Nrf2 and reactive oxygen species. Cells. 11:1266. DOI: 10.3390/cells11081266. PMID: 35455944. PMCID: PMC9028283. PMID: 0a5bd3742e9c463e974182e4f9f9a1ff. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85127911267&origin=inward.
Article12. Brown AR, Alhallak I, Simmen RCM, Melnyk SB, Heard-Lipsmeyer ME, Montales MTE, Habenicht D, Van TT, Simmen FA. 2022; Krüppel-like factor 9 (KLF9) suppresses hepatocellular carcinoma (HCC)-promoting oxidative stress and inflammation in mice fed high-fat diet. Cancers (Basel). 14:1737. DOI: 10.3390/cancers14071737. PMID: 35406507. PMCID: PMC8996893. PMID: ba33486588a341e9b281825eb28d73d2. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85127378750&origin=inward.
Article13. Liu H, Xing R, Ou Z, Zhao J, Hong G, Zhao TJ, Han Y, Chen Y. 2021; G-protein-coupled receptor GPR17 inhibits glioma development by increasing polycomb repressive complex 1-mediated ROS production. Cell Death Dis. 12:610. DOI: 10.1038/s41419-021-03897-0. PMID: 34120140. PMCID: PMC8197764. PMID: c8cd6419e7d149678a495007870fdb39. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107717378&origin=inward.
Article14. Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K. 2020; Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 10:320. DOI: 10.3390/biom10020320. PMID: 32079324. PMCID: PMC7072240. PMID: c40347b987ba4ec39d2e758b5c5e173d. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079636983&origin=inward.
Article15. Hanna A, Shinde AV, Li R, Alex L, Humeres C, Balasubramanian P, Frangogiannis NG. 2021; Collagen denaturation in the infarcted myocardium involves temporally distinct effects of MT1-MMP-dependent proteolysis and mechanical tension. Matrix Biol. 99:18–42. DOI: 10.1016/j.matbio.2021.05.005. PMID: 34048934. PMCID: PMC8591556. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107820624&origin=inward.
Article16. Zhu H, Ding Y, Zhang Y, Ding X, Zhao J, Ouyang W, Gong J, Zou Y, Liu X, Wu W. 2020; CTRP3 induces an intermediate switch of CD14++CD16+ monocyte subset with anti-inflammatory phenotype. Exp Ther Med. 19:2243–2251. DOI: 10.3892/etm.2020.8467. PMID: 32104290. PMCID: PMC7027268.17. Liu C, Fan Z, He D, Chen H, Zhang S, Guo S, Zheng B, Cen H, Zhao Y, Liu H, Wang L. 2022; Designer functional nanomedicine for myocardial repair by regulating the inflammatory microenvironment. Pharmaceutics. 14:758. DOI: 10.3390/pharmaceutics14040758. PMID: 35456592. PMCID: PMC9025700. PMID: 719de61f681c4e298a59d56c4c6d5f72. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85128271826&origin=inward.
Article18. Suku M, Forrester L, Biggs M, Monaghan MG. 2022; Resident macrophages and their potential in cardiac tissue engineering. Tissue Eng Part B Rev. 28:579–591. DOI: 10.1089/ten.teb.2021.0036. PMID: 34088222. PMCID: PMC9242717. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85131903419&origin=inward.
Article19. Niu Y, Bai N, Ma Y, Zhong PY, Shang YS, Wang ZL. 2022; Safety and efficacy of anti-inflammatory therapy in patients with coronary artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 22:84. DOI: 10.1186/s12872-022-02525-9. PMID: 35246052. PMCID: PMC8896203. PMID: 4a11097e484c4ccdb49df00a264eb574. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85125807468&origin=inward.
Article20. Zhang Z, Tang J, Cui X, Qin B, Zhang J, Zhang L, Zhang H, Liu G, Wang W, Zhang J. 2021; New insights and novel therapeutic potentials for macrophages in myocardial infarction. Inflammation. 44:1696–1712. DOI: 10.1007/s10753-021-01467-2. PMID: 33866463. PMCID: PMC8460536. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104612330&origin=inward.
Article21. McCall KD, Muccioli M, Benencia F. 2020; Toll-like receptors signaling in the tumor microenvironment. Adv Exp Med Biol. 1223:81–97. DOI: 10.1007/978-3-030-35582-1_5. PMID: 32030686. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079083418&origin=inward.
Article22. Wahid A, Chen W, Wang X, Tang X. 2021; High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed Pharmacother. 139:111555. DOI: 10.1016/j.biopha.2021.111555. PMID: 33865014. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104137217&origin=inward.
Article23. Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE. 2022; High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology. 30:811–820. DOI: 10.1007/s10787-022-00988-y. PMID: 35471628. PMCID: PMC9040700. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85128840102&origin=inward.
Article24. Mohamed ME, Kandeel M, Abd El-Lateef HM, El-Beltagi HS, Younis NS. 2022; The protective effect of anethole against renal ischemia/reperfusion: the role of the TLR2,4/MYD88/NFκB pathway. Antioxidants (Basel). 11:535. DOI: 10.3390/antiox11030535. PMID: 35326185. PMCID: PMC8944622. PMID: 5bb81a13d06e436e8e3b6f265e70073f. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85126307974&origin=inward.
Article25. Arslan F, Keogh B, McGuirk P, Parker AE. 2010; TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010:704202. DOI: 10.1155/2010/704202. PMID: 20628516. PMCID: PMC2902053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77954555483&origin=inward.
Article26. Spurthi KM, Sarikhani M, Mishra S, Desingu PA, Yadav S, Rao S, Maity S, Tamta AK, Kumar S, Majumdar S, Jain A, Raghuraman A, Khan D, Singh I, Samuel RJ, Ramachandra SG, Nandi D, Sundaresan NR. 2018; Toll-like receptor 2 deficiency hyperactivates the FoxO1 transcription factor and induces aging-associated cardiac dysfunction in mice. J Biol Chem. 293:13073–13089. DOI: 10.1074/jbc.RA118.001880. PMID: 29929978. PMCID: PMC6109936. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052082159&origin=inward.
Article27. McConnell BB, Yang VW. 2010; Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 90:1337–1381. DOI: 10.1152/physrev.00058.2009. PMID: 20959618. PMCID: PMC2975554. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78149481592&origin=inward.
Article28. Bai X, Jiang X, Liu Y, Wang Y, Jiang X, Song G, Qiu H, Zhang Q. 2021; Krüppel-like factor 9 upregulates E-cadherin transcription and represses breast cancer invasion and metastasis. Am J Cancer Res. 11:3660–3673. PMID: 34354866. PMCID: PMC8332869.29. Prosdocimo DA, Sabeh MK, Jain MK. 2015; Kruppel-like factors in muscle health and disease. Trends Cardiovasc Med. 25:278–287. DOI: 10.1016/j.tcm.2014.11.006. PMID: 25528994. PMCID: PMC4422160. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84928762309&origin=inward.
Article30. Safi S, Badshah Y, Shabbir M, Zahra K, Khan K, Dilshad E, Afsar T, Almajwal A, Alruwaili NW, Al-Disi D, Abulmeaty M, Razak S. 2022; Predicting 3D structure, cross talks, and prognostic significance of KLF9 in cervical cancer. Front Oncol. 11:797007. DOI: 10.3389/fonc.2021.797007. PMID: 35047407. PMCID: PMC8761731. PMID: ee967f3c5efb43ffb096fe3c5c91965a. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85123206434&origin=inward.
Article31. Qu R, Liu J, Feng L, Li L, Liu J, Sun F, Sun L. 2022; Down-regulation of KLF9 ameliorates LPS-caused acute lung injury and inflammation in mice via reducing GSDMD expression. Autoimmunity. 55:587–596. DOI: 10.1080/08916934.2022.2114465. PMID: 35993279. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85136510194&origin=inward.
Article32. Li Y, Sun Q, Jiang M, Li S, Zhang J, Xu Z, Guo D, Gu T, Wang B, Xiao L, Zhou T, Zhuo W. 2019; KLF9 suppresses gastric cancer cell invasion and metastasis through transcriptional inhibition of MMP28. FASEB J. 33:7915–7928. DOI: 10.1096/fj.201802531R. PMID: 30913394. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065771274&origin=inward.
Article33. Luo X, Chang S, Xiao S, Peng Y, Gao Y, Hu F, Liang J, Xu Y, Du K, Chen Y, Qin J, Meltzer SJ, Deng S, Feng X, Fan X, Hou G, Jin Z, Zhang X. 2022; PAD4-dependent citrullination of nuclear translocation of GSK3β promotes colorectal cancer progression via the degradation of nuclear CDKN1A. Neoplasia. 33:100835. DOI: 10.1016/j.neo.2022.100835. PMID: 36113195. PMCID: PMC9483803. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85138060659&origin=inward.
Article34. Yan Q, He B, Hao G, Liu Z, Tang J, Fu Q, Jiang CX. 2019; KLF9 aggravates ischemic injury in cardiomyocytes through augmenting oxidative stress. Life Sci. 233:116641. DOI: 10.1016/j.lfs.2019.116641. PMID: 31295469. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071783742&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Myocardial Ischemia and Myocardial Injury Caused by Coronary Vasospasm Associated with Hyperthyroidism
- Ethyl Pyruvate Has Anti-Inflammatory and Delayed Myocardial Protective Effects after Regional Ischemia/Reperfusion Injury
- A Case of Anti-Thrombin III Deficiency Discovered by Myocardial Infarction
- A Case of Hereditary Antithrombin III Deficiency Manifested by Myocardial Infarction and Deep Vein Thrombosis
- Myocardial Longitudinal Strain in Prediction of Heart Failure after Acute Myocardial Infarction