Brain Tumor Res Treat.
2023 Jan;11(1):47-58. 10.14791/btrt.2022.0044.
Brain Invasion and Trends in Molecular Research on Meningioma
- Affiliations
-
- 1Department of Neurosurgery, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea
- 2Division of Neuro Oncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- KMID: 2539329
- DOI: http://doi.org/10.14791/btrt.2022.0044
Abstract
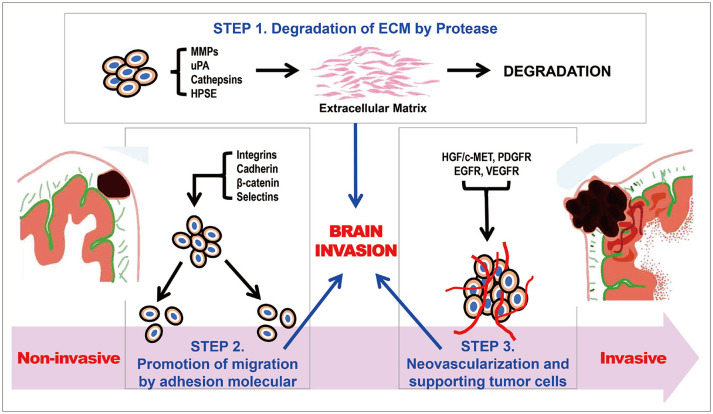
- Meningiomas are the most common primary brain tumors in adults. The treatment of non-benign meningiomas remains a challenging task, and after the publication of the 2021 World Health Organization classification, the importance of molecular biological classification is emerging. In this article, we introduce the mechanisms of brain invasion in atypical meningioma and review the genetic factors involved along with epigenetic regulation. First, it is important to understand the three major steps for brain invasion of meningeal cells: 1) degradation of extracellular matrix by proteases, 2) promotion of tumor cell migration to resident cells by adhesion molecules, and 3) neovascularization and supporting cells by growth factors. Second, the genomic landscape of meningiomas should be analyzed by major categories, such as germline mutations in NF2 and somatic mutations in non-NF2 genes (TRAF7, KLF4, AKT1, SMO, and POLR2A). Finally, epigenetic alterations in meningiomas are being studied, with a focus on DNA methylation, histone modification, and RNA interference. Increasing knowledge of the molecular landscape of meningiomas has allowed the identification of prognostic and predictive markers that can guide therapeutic decision-making processes and the timing of follow-up.
Keyword
Figure
Reference
-
1. Kessler RA, Garzon-Muvdi T, Yang W, Weingart J, Olivi A, Huang J, et al. Metastatic atypical and anaplastic meningioma: a case series and review of the literature. World Neurosurg. 2017; 101:47–56. PMID: 28143726.
Article2. Ruiz J, Martínez A, Hernández S, Zimman H, Ferrer M, Fernández C, et al. Clinicopathological variables, immunophenotype, chromosome 1p36 loss and tumour recurrence of 247 meningiomas grade I and II. Histol Histopathol. 2010; 25:341–349. PMID: 20054806.3. Pizem J, Velnar T, Prestor B, Mlakar J, Popovic M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol. 2014; 33:354–363. PMID: 25034703.
Article4. Sun SQ, Kim AH, Cai C, Murphy RK, DeWees T, Sylvester P, et al. Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery. 2014; 75:347–354. quiz 355. PMID: 24932707.5. Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, et al. Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015; 84:839–845. PMID: 25916182.
Article6. Spille DC, Heß K, Sauerland C, Sanai N, Stummer W, Paulus W, et al. Brain invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg. 2016; 93:346–354. PMID: 27344043.
Article7. Nakasu S, Nakasu Y. Prognostic significance of brain invasion in meningiomas: systematic review and meta-analysis. Brain Tumor Pathol. 2021; 38:81–95. PMID: 33403457.
Article8. Qin C, Huang M, Pan Y, Li Y, Long W, Liu Q. Brain-invasive meningiomas: molecular mechanisms and potential therapeutic options. Brain Tumor Pathol. 2021; 38:156–172. PMID: 33903981.
Article9. Maggio I, Franceschi E, Di Nunno V, Gatto L, Tosoni A, Angelini D, et al. Discovering the molecular landscape of meningioma: the struggle to find new therapeutic targets. Diagnostics (Basel). 2021; 11:1852. PMID: 34679551.
Article10. Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021; 23:1821–1834. PMID: 34181733.11. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022; 128:47–58. PMID: 34633681.
Article12. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
Article13. von Spreckelsen N, Kesseler C, Brokinkel B, Goldbrunner R, Perry A, Mawrin C. Molecular neuropathology of brain-invasive meningiomas. Brain Pathol. 2022; 32:e13048. PMID: 35213084.
Article14. Jalali S, Singh S, Agnihotri S, Wataya T, Salehi F, Alkins R, et al. A role for matrix remodelling proteins in invasive and malignant meningiomas. Neuropathol Appl Neurobiol. 2015; 41:e16–e28. PMID: 24989599.
Article15. Das A, Tan WL, Smith DR. Expression of extracellular matrix markers in benign meningiomas. Neuropathology. 2003; 23:275–281. PMID: 14719542.16. Kandenwein JA, Park-Simon TW, Schramm J, Simon M. uPA/PAI-1 expression and uPA promoter methylation in meningiomas. J Neurooncol. 2011; 103:533–539. PMID: 20862517.17. Lah TT, Nanni I, Trinkaus M, Metellus P, Dussert C, De Ridder L, et al. Toward understanding recurrent meningioma: the potential role of lysosomal cysteine proteases and their inhibitors. J Neurosurg. 2010; 112:940–950. PMID: 19747051.18. Spyrou A, Kundu S, Haseeb L, Yu D, Olofsson T, Dredge K, et al. Inhibition of heparanase in pediatric brain tumor cells attenuates their proliferation, invasive capacity, and in vivo tumor growth. Mol Cancer Ther. 2017; 16:1705–1716. PMID: 28716813.19. Wilisch-Neumann A, Kliese N, Pachow D, Schneider T, Warnke JP, Braunsdorf WE, et al. The integrin inhibitor cilengitide affects meningioma cell motility and invasion. Clin Cancer Res. 2013; 19:5402–5412. PMID: 23948974.20. Zhou K, Wang G, Wang Y, Jin H, Yang S, Liu C. The potential involvement of E-cadherin and beta-catenins in meningioma. PLoS One. 2010; 5:e11231. PMID: 20574529.21. Atukeren P, Turk O, Yanar K, Kemerdere R, Sayyahmelli S, Eren B, et al. Evaluation of ALCAM, PECAM-1 and selectin levels in intracranial meningiomas. Clin Neurol Neurosurg. 2017; 160:21–26. PMID: 28622532.22. Fu J, Su X, Li Z, Deng L, Liu X, Feng X, et al. HGF/c-MET pathway in cancer: from molecular characterization to clinical evidence. Oncogene. 2021; 40:4625–4651. PMID: 34145400.23. Peyre M, Salaud C, Clermont-Taranchon E, Niwa-Kawakita M, Goutagny S, Mawrin C, et al. PDGF activation in PGDS-positive arachnoid cells induces meningioma formation in mice promoting tumor progression in combination with Nf2 and Cdkn2ab loss. Oncotarget. 2015; 6:32713–32722. PMID: 26418719.24. Wang JL, Nistér M, Hermansson M, Westermark B, Pontén J. Expression of PDGF beta-receptors in human meningioma cells. Int J Cancer. 1990; 46:772–778. PMID: 1699901.25. Perry A, Lusis EA, Gutmann DH. Meningothelial hyperplasia: a detailed clinicopathologic, immunohistochemical and genetic study of 11 cases. Brain Pathol. 2005; 15:109–115. PMID: 15912882.26. De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012; 16 Suppl 2:S17–S27.27. Johnson MD, Reeder JE, O’Connell M. MKP-3 regulates PDGF-BB effects and MAPK activation in meningioma cells. J Clin Neurosci. 2015; 22:752–757. PMID: 25698542.28. Wernicke AG, Dicker AP, Whiton M, Ivanidze J, Hyslop T, Hammond EH, et al. Assessment of epidermal growth factor receptor (EGFR) expression in human meningioma. Radiat Oncol. 2010; 5:46. PMID: 20509969.29. Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. 2005; 64:1029–1036. PMID: 16319713.30. Carroll RS, Black PM, Zhang J, Kirsch M, Percec I, Lau N, et al. Expression and activation of epidermal growth factor receptors in meningiomas. J Neurosurg. 1997; 87:315–323. PMID: 9254099.31. Andrae N, Kirches E, Hartig R, Haase D, Keilhoff G, Kalinski T, et al. Sunitinib targets PDGF-receptor and Flt3 and reduces survival and migration of human meningioma cells. Eur J Cancer. 2012; 48:1831–1841. PMID: 22391574.32. de Boüard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007; 9:412–423. PMID: 17622648.33. Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev Anticancer Ther. 2012; 12:567–580. PMID: 22594892.34. Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neurooncol. 2010; 99:365–378. PMID: 20820875.35. Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016; 130:543–552. PMID: 27624915.36. Wahab M, Al-Azzawi F. Meningioma and hormonal influences. Climacteric. 2003; 6:285–292. PMID: 15006250.37. Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008; 26:279–282. PMID: 18182668.38. O’Shea T, Crowley RK, Farrell M, MacNally S, Govender P, Feeney J, et al. Growth of a progesterone receptor-positive meningioma in a female patient with congenital adrenal hyperplasia. Endocrinol Diabetes Metab Case Rep. 2016; 2016:16–0054.39. Benson VS, Kirichek O, Beral V, Green J. Menopausal hormone therapy and central nervous system tumor risk: large UK prospective study and meta-analysis. Int J Cancer. 2015; 136:2369–2377. PMID: 25335165.40. Shu X, Jiang Y, Wen T, Lu S, Yao L, Meng F. Association of hormone replacement therapy with increased risk of meningioma in women: a hospital-based multicenter study with propensity score matching. Asia Pac J Clin Oncol. 2019; 15:e147–e153. PMID: 30761745.41. Qi ZY, Shao C, Huang YL, Hui GZ, Zhou YX, Wang Z. Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS One. 2013; 8:e83261. PMID: 24386167.42. Deli T, Orosz M, Jakab A. Hormone replacement therapy in cancer survivors–review of the literature. Pathol Oncol Res. 2020; 26:63–78. PMID: 30617760.43. Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A, et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006; 24:727–733. PMID: 17162554.44. Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007; 104:10152–10157. PMID: 17551011.45. Giladi M, Schneiderman RS, Voloshin T, Porat Y, Munster M, Blat R, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015; 5:18046. PMID: 26658786.46. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012; 48:2192–2202. PMID: 22608262.47. Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet. 1995; 345:1008–1012. PMID: 7723496.48. Bi WL, Abedalthagafi M, Horowitz P, Agarwalla PK, Mei Y, Aizer AA, et al. Genomic landscape of intracranial meningiomas. J Neurosurg. 2016; 125:525–535. PMID: 26771848.49. Robert SM, Vetsa S, Nadar A, Vasandani S, Youngblood MW, Gorelick E, et al. The integrated multiomic diagnosis of sporadic meningiomas: a review of its clinical implications. J Neurooncol. 2022; 156:205–214. PMID: 34846640.50. Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994; 6:180–184. PMID: 8162072.51. Curto M, McClatchey AI. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer. 2008; 98:256–262. PMID: 17971776.52. Youngblood MW, Duran D, Montejo JD, Li C, Omay SB, Özduman K, et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg. 2020; 133:1345–1354.53. Smith MJ. Germline and somatic mutations in meningiomas. Cancer Genet. 2015; 208:107–114. PMID: 25857641.54. Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013; 45:285–289. PMID: 23334667.55. Boetto J, Bielle F, Sanson M, Peyre M, Kalamarides M. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro Oncol. 2017; 19:345–351. PMID: 28082415.56. Narang A, Maheshwari C, Aggarwal V, Bansal P, Singh P. Gorlin-Goltz syndrome with intracranial meningioma: case report and review of literature. World Neurosurg. 2020; 133:324–330. PMID: 31605858.57. Aizer AA, Abedalthagafi M, Bi WL, Horvath MC, Arvold ND, Al-Mefty O, et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro Oncol. 2016; 18:269–274. PMID: 26323607.58. Shankar GM, Abedalthagafi M, Vaubel RA, Merrill PH, Nayyar N, Gill CM, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017; 19:535–545. PMID: 28170043.59. Williams EA, Wakimoto H, Shankar GM, Barker FG 2nd, Brastianos PK, Santagata S, et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. 2020; 140:89–93. PMID: 32405805.60. Samal S, Patnaik A, Sahu F, Purkait S. Altered expression of epigenetic modifiers EZH2, H3K27me3, and DNA methyltransferases in meningiomas-prognostic biomarkers for routine practice. Folia Neuropathol. 2020; 58:133–142. PMID: 32729292.61. Juratli TA, McCabe D, Nayyar N, Williams EA, Silverman IM, Tummala SS, et al. DMD genomic deletions characterize a subset of progressive/higher-grade meningiomas with poor outcome. Acta Neuropathol. 2018; 136:779–792. PMID: 30123936.62. Sahm F, Schrimpf D, Olar A, Koelsche C, Reuss D, Bissel J, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016; 108:djv377. PMID: 26668184.63. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014; 24:184–189. PMID: 24261697.64. Lu VM, Goyal A, Lee A, Jentoft M, Quinones-Hinojosa A, Chaichana KL. The prognostic significance of TERT promoter mutations in meningioma: a systematic review and meta-analysis. J Neurooncol. 2019; 142:1–10. PMID: 30506498.65. Mirian C, Duun-Henriksen AK, Juratli T, Sahm F, Spiegl-Kreinecker S, Peyre M, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020; 91:378–387. PMID: 32041819.66. Georgescu MM, Nanda A, Li Y, Mobley BC, Faust PL, Raisanen JM, et al. Mutation status and epithelial differentiation stratify recurrence risk in chordoid meningioma—a multicenter study with high prognostic relevance. Cancers (Basel). 2020; 12:225. PMID: 31963394.67. Goutagny S, Yang HW, Zucman-Rossi J, Chan J, Dreyfuss JM, Park PJ, et al. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res. 2010; 16:4155–4164. PMID: 20682713.68. Kim MS, Kim KH, Lee EH, Lee YM, Lee SH, Kim HD, et al. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg. 2014; 121:1189–1200. PMID: 25148008.69. Guyot A, Duchesne M, Robert S, Lia AS, Derouault P, Scaon E, et al. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J Neurooncol. 2019; 145:449–459. PMID: 31729637.70. Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011; 30:E6.71. Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN, et al. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995; 146:827–832. PMID: 7717450.72. Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013; 339:1077–1080. PMID: 23348505.73. James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009; 29:4250–4261. PMID: 19451225.74. James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012; 10:649–659. PMID: 22426462.75. Abedalthagafi M, Bi WL, Aizer AA, Merrill PH, Brewster R, Agarwalla PK, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016; 18:649–655. PMID: 26826201.76. Yuzawa S, Nishihara H, Yamaguchi S, Mohri H, Wang L, Kimura T, et al. Clinical impact of targeted amplicon sequencing for meningioma as a practical clinical-sequencing system. Mod Pathol. 2016; 29:708–716. PMID: 27102344.77. Reuss DE, Piro RM, Jones DT, Simon M, Ketter R, Kool M, et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013; 125:351–358. PMID: 23404370.78. Harmancı AS, Youngblood MW, Clark VE, Coşkun S, Henegariu O, Duran D, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2017; 8:14433. PMID: 28195122.79. Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006; 439:871–874. PMID: 16357870.80. Kheirollahi M, Mehr-Azin M, Kamalian N, Mehdipour P. Expression of cyclin D2, P53, Rb and ATM cell cycle genes in brain tumors. Med Oncol. 2011; 28:7–14. PMID: 20077038.81. Lee Y, Liu J, Patel S, Cloughesy T, Lai A, Farooqi H, et al. Genomic landscape of meningiomas. Brain Pathol. 2010; 20:751–762. PMID: 20015288.82. Spiegl-Kreinecker S, Lötsch D, Neumayer K, Kastler L, Gojo J, Pirker C, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018; 20:1584–1593. PMID: 30010853.83. Yang HW, Kim TM, Song SS, Shrinath N, Park R, Kalamarides M, et al. Alternative splicing of CHEK2 and codeletion with NF2 promote chromosomal instability in meningioma. Neoplasia. 2012; 14:20–28. PMID: 22355270.84. Birzu C, Peyre M, Sahm F. Molecular alterations in meningioma: prognostic and therapeutic perspectives. Curr Opin Oncol. 2020; 32:613–622. PMID: 32890025.85. Collord G, Tarpey P, Kurbatova N, Martincorena I, Moran S, Castro M, et al. An integrated genomic analysis of anaplastic meningioma identifies prognostic molecular signatures. Sci Rep. 2018; 8:13537. PMID: 30202034.86. Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017; 18:682–694. PMID: 28314689.87. Olar A, Wani KM, Wilson CD, Zadeh G, DeMonte F, Jones DT, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017; 133:431–444. PMID: 28130639.88. Nassiri F, Mamatjan Y, Suppiah S, Badhiwala JH, Mansouri S, Karimi S, et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019; 21:901–910. PMID: 31158293.89. Berghoff AS, Hielscher T, Ricken G, Furtner J, Schrimpf D, Widhalm G, et al. Prognostic impact of genetic alterations and methylation classes in meningioma. Brain Pathol. 2022; 32:e12970. PMID: 35213082.90. Kim YZ. Altered histone modifications in gliomas. Brain Tumor Res Treat. 2014; 2:7–21. PMID: 24926467.91. Hansen RS, Gartler SM. 5-Azacytidine-induced reactivation of the human X chromosome-linked PGK1 gene is associated with a large region of cytosine demethylation in the 5’ CpG island. Proc Natl Acad Sci U S A. 1990; 87:4174–4178. PMID: 1693431.92. Ekwall K. Genome-wide analysis of HDAC function. Trends Genet. 2005; 21:608–615. PMID: 16153738.93. Steger DJ, Workman JL. Remodeling chromatin structures for transcription: what happens to the histones? Bioessays. 1996; 18:875–884. PMID: 8939065.94. Lee SH, Lee EH, Lee SH, Lee YM, Kim HD, Kim YZ. Epigenetic role of histone 3 lysine methyltransferase and demethylase in regulating apoptosis predicting the recurrence of atypical meningioma. J Korean Med Sci. 2015; 30:1157–1166. PMID: 26240495.95. Maier AD, Brøchner CB, Mirian C, Haslund-Vinding J, Bartek J Jr, Ekström TJ, et al. Loss of H3K27me3 in WHO grade 3 meningioma. Brain Tumor Pathol. 2022; 39:200–209. PMID: 35678886.96. Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002; 21:5400–5413. PMID: 12154403.97. Katar S, Baran O, Evran S, Cevik S, Akkaya E, Baran G, et al. Expression of miRNA-21, miRNA-107, miRNA-137 and miRNA-29b in meningioma. Clin Neurol Neurosurg. 2017; 156:66–70. PMID: 28349893.98. Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010; 6:e1000879. PMID: 20300657.99. Zhu C, Zhao Y, Zhang Z, Ni Y, Li X, Yong H. MicroRNA-33a inhibits lung cancer cell proliferation and invasion by regulating the expression of β-catenin. Mol Med Rep. 2015; 11:3647–3651. PMID: 25544258.100. Kliese N, Gobrecht P, Pachow D, Andrae N, Wilisch-Neumann A, Kirches E, et al. miRNA-145 is downregulated in atypical and anaplastic meningiomas and negatively regulates motility and proliferation of meningioma cells. Oncogene. 2013; 32:4712–4720. PMID: 23108408.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in Understanding the Molecular Biology of Brain Tumors
- A "Benign" Sphenoid Ridge Meningioma Manifesting as a Subarachnoid Hemorrhage Associated with Tumor Invasion into the Middle Cerebral Artery
- Intraventricular Malignant Meningioma
- Primary Osteolytic Intraosseous Atypical Meningioma with Soft Tissue and Dural Invasion: Report of a Case and Review of Literatures
- A Case of Totally Calcified Meningioma