J Stroke.
2023 Jan;25(1):132-140. 10.5853/jos.2022.02754.
Wall Shear Stress Associated with Stroke Occurrence and Mechanisms in Middle Cerebral Artery Atherosclerosis
- Affiliations
-
- 1Department of Neurology, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 2Department of Radiology, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 3Department of Neurology, Asan Medical Center, Seoul, Korea
- 4Department of Mechanical Engineering & Division of Advanced Nuclear Engineering, POSTECH, Pohang, Korea
- 5Department of Neurology, University of California in Los Angeles, Los Angeles, CA, USA
- KMID: 2539066
- DOI: http://doi.org/10.5853/jos.2022.02754
Abstract
- Background and Purpose
Various mechanisms are involved in the etiology of stroke caused by atherosclerosis of the middle cerebral artery (MCA). Here, we compared differences in plaque nature and hemodynamic parameters according to stroke mechanism in patients with MCA atherosclerosis.
Methods
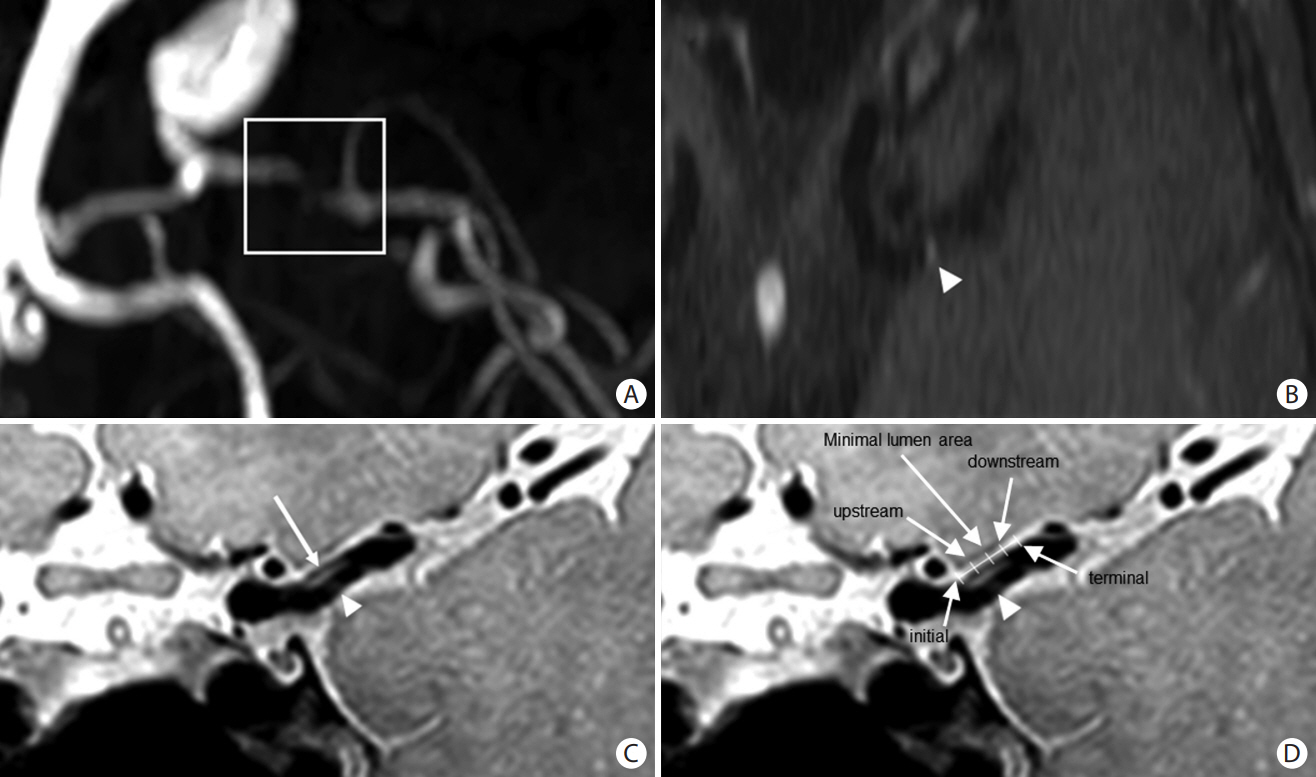
Consecutive patients with asymptomatic and symptomatic MCA atherosclerosis (≥50% stenosis) were enrolled. MCA plaque characteristics (location and plaque enhancement) and wall shear stress (WSS) were measured using high-resolution vessel wall and four-dimensional flow magnetic resonance imaging, respectively, at five points (initial, upstream, minimal lumen, downstream, and terminal). These parameters were compared between patients with asymptomatic and symptomatic MCA atherosclerosis with infarctions of different mechanisms (artery-to-artery embolism vs. local branch occlusion).
Results
In total, 110 patients (46 asymptomatic, 32 artery-to-artery embolisms, and 32 local branch occlusions) were investigated. Plaques were evenly distributed in the MCA of patients with asymptomatic MCA atherosclerosis, more commonly observed in the distal MCA of patients with artery-to-artery embolism, and in the middle MCA of patients with local branch occlusion. Maximum WSS and plaque enhancement were more prominent in the minimum lumen area of patients with asymptomatic MCA atherosclerosis or those with local branch occlusion, and were more prominent in the upstream area in those with artery-to-artery embolism. The elevated variability in the maximum WSS was related to stroke caused by artery-to-artery embolism.
Conclusion
Stroke caused by artery-to-artery embolism was related to plaque enhancement and the highest maximum WSS at the upstream point of the plaque, and was associated with elevated variability of maximum WSS.
Figure
Reference
-
References
1. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012; 43:3313–3318.2. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke. 2014; 16:8–17.3. Tekle WG, Hassan AE. Intracranial atherosclerotic disease: current concepts in medical and surgical management. Neurology. 2021; 97(20 Suppl 2):S145–S157.4. Wu F, Song H, Ma Q, Xiao J, Jiang T, Huang X, et al. Hyperintense plaque on intracranial vessel wall magnetic resonance imaging as a predictor of artery-to-artery embolic infarction. Stroke. 2018; 49:905–911.5. Noh KC, Choi HY, Woo HG, Chang JY, Heo SH, Chang DI, et al. High-on-aspirin platelet reactivity differs between recurrent ischemic stroke associated with extracranial and intracranial atherosclerosis. J Clin Neurol. 2022; 18:421–427.6. Ha SH, Chang JY, Lee SH, Lee KM, Heo SH, Chang DI, et al. Mechanism of stroke according to the severity and location of atherosclerotic middle cerebral artery disease. J Stroke Cerebrovasc Dis. 2021; 30:105503.7. Li ZY, Taviani V, Tang T, Sadat U, Young V, Patterson A, et al. The mechanical triggers of plaque rupture: shear stress vs pressure gradient. Br J Radiol. 2009; 82 Spec No 1:S39–S45.8. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382:1329–1340.9. Kim BJ, Yoon Y, Lee DH, Kang DW, Kwon SU, Kim JS. The shape of middle cerebral artery and plaque location: high-resolution MRI finding. Int J Stroke. 2015; 10:856–860.10. Dhawan SS, Avati Nanjundappa RP, Branch JR, Taylor WR, Quyyumi AA, Jo H, et al. Shear stress and plaque development. Expert Rev Cardiovasc Ther. 2010; 8:545–556.11. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985; 5:293–302.12. Cicha I, Wörner A, Urschel K, Beronov K, Goppelt-Struebe M, Verhoeven E, et al. Carotid plaque vulnerability: a positive feedback between hemodynamic and biochemical mechanisms. Stroke. 2011; 42:3502–3510.13. Park ST, Kim JK, Yoon KH, Park SO, Park SW, Kim JS, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. AJNR Am J Neuroradiol. 2010; 31:1106–1112.14. Woo HG, Heo SH, Kim EJ, Chang DI, Song TJ, Kim BJ. Atherosclerotic plaque locations may be related to different ischemic lesion patterns. BMC Neurol. 2020; 20:288.15. Zhang M, Peng F, Li Y, He L, Liu A, Li R. Associations between morphology and hemodynamics of intracranial aneurysms based on 4D flow and black-blood magnetic resonance imaging. Quant Imaging Med Surg. 2021; 11:597–607.16. Gaidzik F, Pravdivtseva M, Larsen N, Jansen O, Hövener JB, Berg P. Luminal enhancement in intracranial aneurysms: fact or feature?-A quantitative multimodal flow analysis. Int J Comput Assist Radiol Surg. 2021; 16:1999–2008.17. Sekine T, Takagi R, Amano Y, Murai Y, Orita E, Fukushima Y, et al. 4D flow MR imaging of ophthalmic artery flow in patients with internal carotid artery stenosis. Magn Reson Med Sci. 2018; 17:13–20.18. Ando T, Sekine T, Murai Y, Orita E, Takagi R, Amano Y, et al. Multiparametric flow analysis using four-dimensional flow magnetic resonance imaging can detect cerebral hemodynamic impairment in patients with internal carotid artery stenosis. Neuroradiology. 2020; 62:1421–1431.19. Youn SW, Lee J. From 2D to 4D phase-contrast MRI in the neurovascular system: will it be a quantum jump or a fancy decoration? J Magn Reson Imaging. 2022; 55:347–372.20. Ko Y, Lee S, Chung JW, Han MK, Park JM, Kang K, et al. MRI-based algorithm for acute ischemic stroke subtype classification. J Stroke. 2014; 16:161–172.21. Chen Z, Qin H, Liu J, Wu B, Cheng Z, Jiang Y, et al. Characteristics of wall shear stress and pressure of intracranial atherosclerosis analyzed by a computational fluid dynamics model: a pilot study. Front Neurol. 2019; 10:1372.22. Lee JM, Choi G, Hwang D, Park J, Kim HJ, Doh JH, et al. Impact of longitudinal lesion geometry on location of plaque rupture and clinical presentations. JACC Cardiovasc Imaging. 2017; 10:677–688.23. Wang E, Shao S, Li S, Yan P, Xiang Y, Wang X, et al. A high-resolution MRI study of the relationship between plaque enhancement and ischemic stroke events in patients with intracranial atherosclerotic stenosis. Front Neurol. 2018; 9:1154.24. Kim JM, Jung KH, Sohn CH, Moon J, Shin JH, Park J, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke. 2016; 11:171–179.25. Eshtehardi P, Brown AJ, Bhargava A, Costopoulos C, Hung OY, Corban MT, et al. High wall shear stress and high-risk plaque: an emerging concept. Int J Cardiovasc Imaging. 2017; 33:1089–1099.26. Corban MT, Eshtehardi P, Suo J, McDaniel MC, Timmins LH, Rassoul-Arzrumly E, et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014; 232:271–276.27. Groen HC, Gijsen FJ, van der Lugt A, Ferguson MS, Hatsukami TS, van der Steen AF, et al. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke. 2007; 38:2379–2381.28. de Weert TT, Cretier S, Groen HC, Homburg P, Cakir H, Wentzel JJ, et al. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke. 2009; 40:1334–1340.29. Thondapu V, Mamon C, Poon EKW, Kurihara O, Kim HO, Russo M, et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res. 2021; 117:1974–1985.30. Leng X, Lan L, Ip HL, Abrigo J, Scalzo F, Liu H, et al. Hemodynamics and stroke risk in intracranial atherosclerotic disease. Ann Neurol. 2019; 85:752–764.31. Rana A, Westein E, Niego B, Hagemeyer CE. Shear-dependent platelet aggregation: mechanisms and therapeutic opportunities. Front Cardiovasc Med. 2019; 6:141.32. Ha H, Lee SJ. Hemodynamic features and platelet aggregation in a stenosed microchannel. Microvasc Res. 2013; 90:96–105.33. Zhao YC, Vatankhah P, Goh T, Michelis R, Kyanian K, Zhang Y, et al. Hemodynamic analysis for stenosis microfluidic model of thrombosis with refined computational fluid dynamics simulation. Sci Rep. 2021; 11:6875.34. Chen Y, Liu J, Li M, Yu Y, Yan Z, Shiu W, et al. Non-invasive assessment of intracranial wall shear stress using high-resolution magnetic resonance imaging in combination with computational fluid dynamics technique. Fundam Res. 2022; 2:329–334.35. Dolan JM, Kolega J, Meng H. High wall shear stress and spatial gradients in vascular pathology: a review. Ann Biomed Eng. 2013; 41:1411–1427.36. Sing CE, Alexander-Katz A. Elongational flow induces the unfolding of von Willebrand factor at physiological flow rates. Biophys J. 2010; 98:L35–L37.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between Ischemic Stroke and Vascular Shear Stress in the Carotid Artery
- Intracranial Cerebrovascular Revascularization(Extracranial-Intracranial Arterial Bypass, EIAB)
- Vessel Wall Imaging of the Intracranial and Cervical Carotid Arteries
- High Shear Stress at the Surface of Enhancing Plaque in the Systolic Phase is Related to the Symptom Presentation of Severe M1 Stenosis
- Moyamoya-like Disease