J Pathol Transl Med.
2023 Jan;57(1):28-42. 10.4132/jptm.2022.11.14.
Infections and immunity: associations with obesity and related metabolic disorders
- Affiliations
-
- 1College of Medical Science, Alderson Broaddus University, Philippi, WV, USA
- 2Division of Research and Development, Hormel Foods Corporation, Austin, MN, USA
- 3Division of Medical & Behavioral Health, Pueblo Community College, Pueblo, CO, USA
- 4WuXi AppTec, St. Paul, MN, USA
- 5Lake Erie College of Osteopathic Medicine, Bradenton, FL, USA
- KMID: 2539034
- DOI: http://doi.org/10.4132/jptm.2022.11.14
Abstract
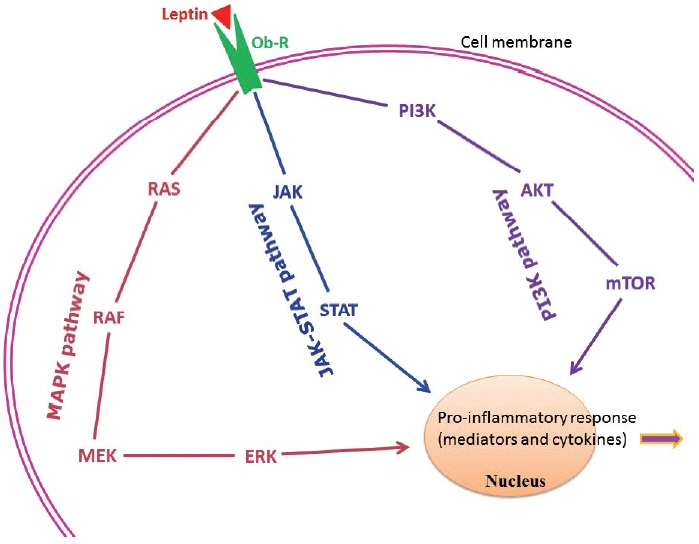
- About one-fourth of the global population is either overweight or obese, both of which increase the risk of insulin resistance, cardiovascular diseases, and infections. In obesity, both immune cells and adipocytes produce an excess of pro-inflammatory cytokines that may play a significant role in disease progression. In the recent coronavirus disease 2019 (COVID-19) pandemic, important pathological characteristics such as involvement of the renin-angiotensin-aldosterone system, endothelial injury, and pro-inflammatory cytokine release have been shown to be connected with obesity and associated sequelae such as insulin resistance/type 2 diabetes and hypertension. This pathological connection may explain the severity of COVID-19 in patients with metabolic disorders. Many studies have also reported an association between type 2 diabetes and persistent viral infections. Similarly, diabetes favors the growth of various microorganisms including protozoal pathogens as well as opportunistic bacteria and fungi. Furthermore, diabetes is a risk factor for a number of prion-like diseases. There is also an interesting relationship between helminths and type 2 diabetes; helminthiasis may reduce the pro-inflammatory state, but is also associated with type 2 diabetes or even neoplastic processes. Several studies have also documented altered circulating levels of neutrophils, lymphocytes, and monocytes in obesity, which likely modifies vaccine effectiveness. Timely monitoring of inflammatory markers (e.g., C-reactive protein) and energy homeostasis markers (e.g., leptin) could be helpful in preventing many obesity-related diseases.
Keyword
Figure
Reference
-
References
1. Gammone MA, D’Orazio N. COVID-19 and obesity: overlapping of two pandemics. Obes Facts. 2021; 14:579–85.
Article2. Sattar N, Valabhji J. Obesity as a risk factor for severe COVID-19: summary of the best evidence and implications for health care. Curr Obes Rep. 2021; 10:282–9.
Article3. Dayaramani C, De Leon J, Reiss AB. Cardiovascular disease complicating COVID-19 in the elderly. Medicina (Kaunas). 2021; 57:833.
Article4. Ghilotti F, Bellocco R, Ye W, Adami HO, Trolle Lagerros Y. Obesity and risk of infections: results from men and women in the Swedish National March Cohort. Int J Epidemiol. 2019; 48:1783–94.
Article5. Dhurandhar NV, Bailey D, Thomas D. Interaction of obesity and infections. Obes Rev. 2015; 16:1017–29.
Article6. Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018; 104:525–34.
Article7. Chakraborty S, Bhattacharyya R, Banerjee D. Infections: a possible risk factor for type 2 diabetes. Adv Clin Chem. 2017; 80:227–51.8. de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012; 71:332–8.
Article9. Cinkajzlova A, Mraz M, Haluzik M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J Endocrinol. 2021; 252:R1–22.10. Iskander K, Farhour R, Ficek M, Ray A. Obesity-related complications: few biochemical phenomena with reference to tumorigenesis. Malays J Pathol. 2013; 35:1–15.11. Ray A. Cancer and comorbidity: the role of leptin in breast cancer and associated pathologies. World J Clin Cases. 2018; 6:483–92.
Article12. Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci (Lond). 2021; 135:731–52.
Article13. Fernandez-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010; 2010:568343.14. Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005; 116:1228–34.
Article15. Ahmetaj-Shala B, Vaja R, Atanur SS, George PM, Kirkby NS, Mitchell JA. Cardiorenal tissues express SARS-CoV-2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC Basic Transl Sci. 2020; 5:1111–23.
Article16. Lubbe L, Cozier GE, Oosthuizen D, Acharya KR, Sturrock ED. ACE2 and ACE: structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin Sci (Lond). 2020; 134:2851–71.
Article17. Wong MK. Angiotensin converting enzymes. In : Takei Y, Ando H, Tsutsui K, editors. Handbook of hormones. Oxford: Elsevier Academic Press;2016. p. 263–e29D-4.18. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013; 77:301–8.
Article19. Varga Z. Endotheliitis in COVID-19. Pathologe. 2020; 41(Suppl 2):99–102.20. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–8.
Article21. Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020; 19:100283.
Article22. Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: impact of obesity and diabetes on disease severity. Clin Obes. 2020; 10:e12414.23. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020; 371:m3862.
Article24. Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARSCoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blockerbased cardiovascular therapies. Intern Emerg Med. 2020; 15:759–66.
Article25. Halim AA, Alsayed B, Embarak S, Yaseen T, Dabbous S. Clinical characteristics and outcome of ICU admitted MERS corona virus infected patients. Egypt J Chest Dis Tuberc. 2016; 65:81–7.
Article26. Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016; 49:129–33.
Article27. Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019; 10:1071.
Article28. Bhattacharya I, Ghayor C, Perez Dominguez A, Weber FE. From influenza virus to novel corona virus (SARS-CoV-2): the contribution of obesity. Front Endocrinol (Lausanne). 2020; 11:556962.29. Ndako JA, Nwankiti OO, Olorundare JO, et al. Studies on the serological markers for hepatitis B virus infection among type 2 diabetic patients. J Clin Lab Anal. 2021; 35:e23464.
Article30. Iovanescu VF, Streba CT, Ionescu M, et al. Diabetes mellitus and renal involvement in chronic viral liver disease. J Med Life. 2015; 8:483–7.31. Cheng AY, Kong AP, Wong VW, et al. Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia. 2006; 49:1777–84.
Article32. Virseda Chamorro I, Virseda Chamorro M, Prieto Carbajo RI, Jaqueti Aroca J. Hepatitis C as a risk factor of diabetes mellitus type 2. Rev Clin Esp. 2006; 206:167–71.33. Arao M, Murase K, Kusakabe A, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003; 38:355–60.
Article34. Dworzanski J, Drop B, Kliszczewska E, Strycharz-Dudziak M, Polz-Dacewicz M. Prevalence of Epstein-Barr virus, human papillomavirus, cytomegalovirus and herpes simplex virus type 1 in patients with diabetes mellitus type 2 in south-eastern Poland. PLoS One. 2019; 14:e0222607.
Article35. Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007-2008. PLoS One. 2011; 6:e19092.
Article36. Fernandez-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring). 2007; 15:245–52.
Article37. Sun YH, Pei WD, Wu YJ, Wang GG. Association of herpes simplex virus type2 infection with dyslipidemia in Chinese. Zhonghua Yi Xue Za Zhi. 2003; 83:1774–7.38. Woelfle T, Linkohr B, Waterboer T, et al. Health impact of seven herpesviruses on (pre)diabetes incidence and HbA(1c): results from the KORA cohort. Diabetologia. 2022; 65:1328–38.
Article39. Yoo SG, Han KD, Lee KH, La Y, Kwon DE, Han SH. Impact of cytomegalovirus disease on new-onset type 2 diabetes mellitus: population-based matched case-control cohort study. Diabetes Metab J. 2019; 43:815–29.
Article40. Chen S, de Craen AJ, Raz Y, et al. Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old: results from the Leiden 85-plus study. Immun Ageing. 2012; 9:18.
Article41. Roberts BW, Cech I. Association of type 2 diabetes mellitus and seroprevalence for cytomegalovirus. South Med J. 2005; 98:686–92.
Article42. Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997; 96:2144–8.
Article43. Reinholdt K, Thomsen LT, Munk C, et al. Incidence of HPV-related anogenital intraepithelial neoplasia and cancer in men with diabetes compared with the general population. Epidemiology. 2021; 32:705–11.
Article44. Sobti A, Sharif-Askari FS, Khan S, et al. Logistic regression prediction model identify type 2 diabetes mellitus as a prognostic factor for human papillomavirus-16 associated head and neck squamous cell carcinoma. PLoS One. 2019; 14:e0217000.
Article45. Slama L, Barrett BW, Abraham AG, et al. Risk for incident diabetes is greater in prediabetic men with HIV than without HIV. AIDS. 2021; 35:1605–14.
Article46. Kubiak RW, Kratz M, Motala AA, et al. Clinic-based diabetes screening at the time of HIV testing and associations with poor clinical outcomes in South Africa: a cohort study. BMC Infect Dis. 2021; 21:789.
Article47. Hema A, Poda A, Tougouma JB, et al. Diabetes mellitus and high blood pressure over risk in HIV-infected people followed at Souro Sanou University Hospital Day Hospital, Bobo-Dioulasso 2018. Rev Epidemiol Sante Publique. 2021; 69:72–7.48. Jeremiah K, Filteau S, Faurholt-Jepsen D, et al. Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV-infected and uninfected Tanzanian adults. PLoS One. 2020; 15:e0230723.
Article49. Albashir AA. The potential impacts of obesity on COVID-19. Clin Med (Lond). 2020; 20:e109–13.
Article50. Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a Clinician’s perspective. Diabetes Metab Syndr. 2020; 14:971–8.
Article51. Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020; 26:1395–9.
Article52. Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect. 2021; 27:9–11.
Article53. Cafardi J, Haas D, Lamarre T, Feinberg J. Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis. 2021; 8:ofab016.
Article54. Taylor M, Ghodasara A, Ismail A, Gauhar U, El-Kersh K. Disseminated histoplasmosis in an immunocompetent patient after COVID-19 pneumonia. Cureus. 2021; 13:e17269.
Article55. Amin A, Vartanian A, Poladian N, et al. Root causes of fungal coinfections in COVID-19 infected patients. Infect Dis Rep. 2021; 13:1018–35.
Article56. Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020; 39:101044.
Article57. Choudhary NK, Jain AK, Soni R, Gahlot N. Mucormycosis: a deadly black fungus infection among COVID-19 patients in India. Clin Epidemiol Glob Health. 2021; 12:100900.
Article58. Kuchi Bhotla H, Balasubramanian B, Meyyazhagan A, et al. Opportunistic mycoses in COVID-19 patients/survivors: epidemic inside a pandemic. J Infect Public Health. 2021; 14:1720–6.
Article59. Speth C, Rambach G, Wurzner R, Lass-Florl C. Complement and fungal pathogens: an update. Mycoses. 2008; 51:477–96.
Article60. Soni S, Namdeo Pudake R, Jain U, Chauhan N. A systematic review on SARS-CoV-2-associated fungal coinfections. J Med Virol. 2022; 94:99–109.61. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982; 216:136–44.
Article62. Linden R. The biological function of the prion protein: a cell surface scaffold of signaling modules. Front Mol Neurosci. 2017; 10:77.
Article63. Sikorska B, Liberski PP. Human prion diseases: from Kuru to variant Creutzfeldt-Jakob disease. Subcell Biochem. 2012; 65:457–96.
Article64. Aguilar-Calvo P, Garcia C, Espinosa JC, Andreoletti O, Torres JM. Prion and prion-like diseases in animals. Virus Res. 2015; 207:82–93.
Article65. Duyckaerts C, Clavaguera F, Potier MC. The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr Opin Neurol. 2019; 32:266–71.
Article66. Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol. 2018; 19:755–73.
Article67. Lee J, Kim SY, Hwang KJ, Ju YR, Woo HJ. Prion diseases as transmissible zoonotic diseases. Osong Public Health Res Perspect. 2013; 4:57–66.
Article68. Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. 2018; 19:3306.
Article69. Kellett KA, Hooper NM. Prion protein and Alzheimer disease. Prion. 2009; 3:190–4.
Article70. Sitammagari KK, Masood W, et al. Creutzfeldt Jakob disease [Internet]. Treasure Island: StatPearls Publishing. 2022 [cited 2022 Mar 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507860/.71. Collinge J, Whitfield J, McKintosh E, et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci. 2008; 363:3725–39.
Article72. Guo Y, Xu Y, Lin X, et al. Creutzfeldt-Jakob disease: alterations of gut microbiota. Front Neurol. 2022; 13:832599.
Article73. Yang D, Zhao D, Shah SZA, et al. Implications of gut microbiota dysbiosis and metabolic changes in prion disease. Neurobiol Dis. 2020; 135:104704.
Article74. Fang P, Kazmi SA, Jameson KG, Hsiao EY. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe. 2020; 28:201–22.
Article75. Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol. 2020; 57:5026–43.
Article76. Nishiwaki H, Ito M, Ishida T, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord. 2020; 35:1626–35.
Article77. Gasmi Benahmed A, Gasmi A, Dosa A, et al. Association between the gut and oral microbiome with obesity. Anaerobe. 2021; 70:102248.
Article78. Thomas MS, Blesso CN, Calle MC, Chun OK, Puglisi M, Fernandez ML. Dietary influences on gut microbiota with a focus on metabolic syndrome. Metab Syndr Relat Disord. 2022; 20:429–39.
Article79. Gradisteanu Pircalabioru G, Liaw J, Gundogdu O, et al. Effects of the lipid profile, type 2 diabetes and medication on the metabolic syndrome-associated gut microbiome. Int J Mol Sci. 2022; 23:7509.
Article80. Bielka W, Przezak A, Pawlik A. The role of the gut microbiota in the pathogenesis of diabetes. Int J Mol Sci. 2022; 23:480.
Article81. Gomez-Gutierrez R, Morales R. The prion-like phenomenon in Alzheimer’s disease: evidence of pathology transmission in humans. PLoS Pathog. 2020; 16:e1009004.
Article82. Zhou J, Liu B. Alzheimer’s disease and prion protein. Intractable Rare Dis Res. 2013; 2:35–44.
Article83. Soto C. Transmissible proteins: expanding the prion heresy. Cell. 2012; 149:968–77.
Article84. Liberski PP, Gajos A, Sikorska B, Lindenbaum S. Kuru, the first human prion disease. Viruses. 2019; 11:232.
Article85. Brandner S, Jaunmuktane Z. Prion disease: experimental models and reality. Acta Neuropathol. 2017; 133:197–222.
Article86. Manuelidis L, Chakrabarty T, Miyazawa K, Nduom NA, Emmerling K. The kuru infectious agent is a unique geographic isolate distinct from Creutzfeldt-Jakob disease and scrapie agents. Proc Natl Acad Sci U S A. 2009; 106:13529–34.
Article87. Ayers JI, Brooks MM, Rutherford NJ, et al. Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J Virol. 2017; 91:e02095–16.88. Betemps D, Verchere J, Brot S, et al. Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological alpha-synuclein by enhanced ELISA. Acta Neuropathol Commun. 2014; 2:29.89. Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015; 129:221–37.
Article90. Guo JL, Narasimhan S, Changolkar L, et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016; 213:2635–54.
Article91. Lam S, Petit F, Herard AS, et al. Transmission of amyloid-beta and tau pathologies is associated with cognitive impairments in a primate. Acta Neuropathol Commun. 2021; 9:165.
Article92. Morales R, Bravo-Alegria J, Duran-Aniotz C, Soto C. Titration of biologically active amyloid-beta seeds in a transgenic mouse model of Alzheimer’s disease. Sci Rep. 2015; 5:9349.93. Wagner J, Ryazanov S, Leonov A, et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013; 125:795–813.
Article94. Wagner J, Krauss S, Shi S, et al. Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies. Acta Neuropathol. 2015; 130:619–31.
Article95. Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020; 16:e1008508.
Article96. Khudhair Z, Alhallaf R, Eichenberger RM, et al. Gastrointestinal helminth infection improves insulin sensitivity, decreases systemic inflammation, and alters the composition of gut microbiota in distinct mouse models of type 2 diabetes. Front Endocrinol (Lausanne). 2020; 11:606530.
Article97. Hays R, Esterman A, Giacomin P, Loukas A, McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract. 2015; 107:355–61.
Article98. Rajamanickam A, Munisankar S, Bhootra Y, et al. Metabolic consequences of concomitant Strongyloides stercoralis infection in patients with type 2 diabetes mellitus. Clin Infect Dis. 2019; 69:697–704.
Article99. Rajamanickam A, Munisankar S, Dolla C, et al. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis. 2020; 14:e0008101.
Article100. Muthukumar R, Suttiprapa S, Mairiang E, et al. Effects of Opisthorchis viverrini infection on glucose and lipid profiles in human hosts: a cross-sectional and prospective follow-up study from Thailand. Parasitol Int. 2020; 75:102000.101. Htun NS, Odermatt P, Paboriboune P, et al. Association between helminth infections and diabetes mellitus in adults from the Lao People’s Democratic Republic: a cross-sectional study. Infect Dis Poverty. 2018; 7:105.
Article102. Moudgil V, Rana R, Tripathi PK, Farooq U, Sehgal R, Khan MA. Coprevalence of parasitic infections and diabetes in Sub-Himalayan region of Northern India. Int J Health Sci (Qassim). 2019; 13:19–24.103. Ambachew S, Assefa M, Tegegne Y, Zeleke AJ. The prevalence of intestinal parasites and their associated factors among diabetes mellitus patients at the University of Gondar Referral Hospital, northwest Ethiopia. J Parasitol Res. 2020; 2020:8855965.
Article104. Almugadam BS, Ibrahim MK, Liu Y, et al. Association of urogenital and intestinal parasitic infections with type 2 diabetes individuals: a comparative study. BMC Infect Dis. 2021; 21:20.
Article105. Udoh BE, Iwalokun BA, Etukumana E, Amoo J. Asymptomatic falciparum malaria and its effects on type 2 diabetes mellitus patients in Lagos, Nigeria. Saudi J Med Med Sci. 2020; 8:32–40.
Article106. Wyss K, Wangdahl A, Vesterlund M, et al. Obesity and diabetes as risk factors for severe Plasmodium falciparum malaria: results from a Swedish nationwide study. Clin Infect Dis. 2017; 65:949–58.107. Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010; 16:1601–4.
Article108. Vizzoni AG, Varela MC, Sangenis LH, Hasslocher-Moreno AM, do Brasil P, Saraiva RM. Ageing with Chagas disease: an overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors. 2018; 11:354.
Article109. dos Santos VM, da Cunha SF, Teixeira Vde P, et al. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev Soc Bras Med Trop. 1999; 32:489–96.110. Soltani S, Tavakoli S, Sabaghan M, Kahvaz MS, Pashmforosh M, Foroutan M. The probable association between chronic Toxoplasma gondii infection and type 1 and type 2 diabetes mellitus: a casecontrol study. Interdiscip Perspect Infect Dis. 2021; 2021:2508780.111. Li YX, Xin H, Zhang XY, et al. Toxoplasma gondii infection in diabetes mellitus patients in China: seroprevalence, risk factors, and case-control studies. Biomed Res Int. 2018; 2018:4723739.112. Reeves GM, Mazaheri S, Snitker S, et al. A Positive association between T. gondii seropositivity and obesity. Front Public Health. 2013; 1:73.113. Machado ER, Matos NO, Rezende SM, et al. Host-parasite interactions in individuals with type 1 and 2 diabetes result in higher frequency of Ascaris lumbricoides and Giardia lamblia in type 2 diabetic individuals. J Diabetes Res. 2018; 2018:4238435.114. Sisu A, Abugri J, Ephraim RK, et al. Intestinal parasite infections in diabetes mellitus patients: a cross-sectional study of the Bolgatanga municipality, Ghana. Sci Afr. 2021; 11:e00680.
Article115. Akinbo FO, Olujobi SO, Omoregie R, Egbe C. Intestinal parasitic infections among diabetes mellitus patients. Biomark Genom Med. 2013; 5:44–7.
Article116. Alemu G, Jemal A, Zerdo Z. Intestinal parasitosis and associated factors among diabetic patients attending Arba Minch Hospital, Southern Ethiopia. BMC Res Notes. 2018; 11:689.
Article117. van Tong H, Brindley PJ, Meyer CG, Velavan TP. Parasite infection, carcinogenesis and human malignancy. EBioMedicine. 2017; 15:12–23.
Article118. Muehlenbachs A, Bhatnagar J, Agudelo CA, et al. Malignant transformation of Hymenolepis nana in a human host. N Engl J Med. 2015; 373:1845–52.
Article119. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011; 54:173–84.
Article120. Huang MH, Chen CH, Yen CM, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. 2005; 20:141–6.
Article121. Chaidee A, Onsurathum S, Intuyod K, et al. Co-occurrence of opisthorchiasis and diabetes exacerbates morbidity of the hepatobiliary tract disease. PLoS Negl Trop Dis. 2018; 12:e0006611.
Article122. Thinkhamrop K, Khuntikeo N, Laohasiriwong W, Chupanit P, Kelly M, Suwannatrai AT. Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl Trop Dis. 2021; 15:e0009741.123. Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol. 2020; 15:179–209.
Article124. Shibuya T. Eosinophilic response in parasitic diseases. Nihon Rinsho. 1993; 51:825–31.125. Linnemann LC, Reitz M, Feyerabend TB, Breloer M, Hartmann W. Limited role of mast cells during infection with the parasitic nematode Litomosoides sigmodontis. PLoS Negl Trop Dis. 2020; 14:e0008534.126. Lu F, Huang S. The roles of mast cells in parasitic protozoan infections. Front Immunol. 2017; 8:363.
Article127. Mitre E, Nutman TB. Lack of basophilia in human parasitic infections. Am J Trop Med Hyg. 2003; 69:87–91.
Article128. Rosales C. Neutrophils vs. amoebas: immunity against the protozoan parasite Entamoeba histolytica. J Leukoc Biol. 2021; 110:1241–52.
Article129. Passelli K, Billion O, Tacchini-Cottier F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front Immunol. 2021; 12:649348.
Article130. Aitken EH, Alemu A, Rogerson SJ. Neutrophils and malaria. Front Immunol. 2018; 9:3005.
Article131. Marzullo P, Minocci A, Giarda P, et al. Lymphocytes and immunoglobulin patterns across the threshold of severe obesity. Endocrine. 2014; 45:392–400.
Article132. Yoshimura A, Ohnishi S, Orito C, et al. Association of peripheral total and differential leukocyte counts with obesity-related complications in young adults. Obes Facts. 2015; 8:1–16.
Article133. Erdal E, Inanir M. Platelet-to-lymphocyte ratio (PLR) and plateletcrit (PCT) in young patients with morbid obesity. Rev Assoc Med Bras (1992). 2019; 65:1182–7.
Article134. Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg. 2006; 16:251–7.
Article135. Xu X, Su S, Wang X, et al. Obesity is associated with more activated neutrophils in African American male youth. Int J Obes (Lond). 2015; 39:26–32.
Article136. Raghavan V, Gunasekar D, Rao KR. Relevance of haematologic parameters in obese women with or without metabolic syndrome. J Clin Diagn Res. 2016; 10:EC11–6.
Article137. Brotfain E, Hadad N, Shapira Y, et al. Neutrophil functions in morbidly obese subjects. Clin Exp Immunol. 2015; 181:156–63.
Article138. Nieman DC, Henson DA, Nehlsen-Cannarella SL, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999; 99:294–9.
Article139. Womack J, Tien PC, Feldman J, et al. Obesity and immune cell counts in women. Metabolism. 2007; 56:998–1004.
Article140. Al-Sufyani AA, Mahassni SH. Obesity and immune cells in Saudi females. Innate Immun. 2011; 17:439–50.
Article141. Furuncuoglu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016; 20:1300–6.142. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021; 122:474–88.
Article143. Karakaya S, Altay M, Kaplan Efe F, et al. The neutrophil-lymphocyte ratio and its relationship with insulin resistance in obesity. Turk J Med Sci. 2019; 49:245–8.
Article144. Kim DJ, Noh JH, Lee BW, et al. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J Korean Med Sci. 2008; 23:193–8.
Article145. Yu JY, Choi WJ, Lee HS, Lee JW. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: an observational study. Medicine (Baltimore). 2019; 98:e14740.146. Syauqy A, Hsu CY, Rau HH, Chao JC. Association of dietary patterns, anthropometric measurements, and metabolic parameters with C-reactive protein and neutrophil-to-lymphocyte ratio in middle-aged and older adults with metabolic syndrome in Taiwan: a cross-sectional study. Nutr J. 2018; 17:106.
Article147. Bahadir A, Baltaci D, Turker Y, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol J Cardiol. 2015; 15:816–22.
Article148. Yilmaz H, Ucan B, Sayki M, et al. Usefulness of the neutrophil-tolymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr. 2015; 9:299–304.
Article149. Thavaraputta S, Dennis JA, Ball S, Laoveeravat P, Nugent K. Relation of hematologic inflammatory markers and obesity in otherwise healthy participants in the National Health and Nutrition Examination Survey, 2011-2016. Proc (Bayl Univ Med Cent). 2020; 34:17–21.
Article150. Ip BC, Hogan AE, Nikolajczyk BS. Lymphocyte roles in metabolic dysfunction: of men and mice. Trends Endocrinol Metab. 2015; 26:91–100.
Article151. Bahr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018; 66:234–44.
Article152. Rodriguez CP, Gonzalez MC, Aguilar-Salinas CA, Najera-Medina O. Peripheral lymphocytes, obesity, and metabolic syndrome in young adults: an immunometabolism study. Metab Syndr Relat Disord. 2018; 16:342–9.
Article153. Sato Mito N, Suzui M, Yoshino H, Kaburagi T, Sato K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging. 2009; 13:602–6.
Article154. Breznik JA, Foley KP, Maddiboina D, Schertzer JD, Sloboda DM, Bowdish DM. Effects of obesity-associated chronic inflammation on peripheral blood immunophenotype are not mediated by TNF in female C57BL/6J mice. Immunohorizons. 2021; 5:370–83.
Article155. Tenorio TR, Farah BQ, Ritti-Dias RM, et al. Relation between leukocyte count, adiposity, and cardiorespiratory fitness in pubertal adolescents. Einstein (Sao Paulo). 2014; 12:420–4.
Article156. Friedrich K, Sommer M, Strobel S, et al. Perturbation of the monocyte compartment in human obesity. Front Immunol. 2019; 10:1874.
Article157. Christou KA, Christou GA, Karamoutsios A, et al. Metabolically healthy obesity is characterized by a proinflammatory phenotype of circulating monocyte subsets. Metab Syndr Relat Disord. 2019; 17:259–65.
Article158. Subramanian V, Ferrante AW Jr. Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program. 2009; 63:151–9.
Article159. Bastarrachea RA, Lopez-Alvarenga JC, Bolado-Garcia VE, TellezMendoza J, Laviada-Molina H, Comuzzie AG. Macrophages, inflammation, adipose tissue, obesity and insulin resistance. Gac Med Mex. 2007; 143:505–12.160. Shehata HM, Murphy AJ, Lee MK, et al. Sugar or fat?: metabolic requirements for immunity to viral infections. Front Immunol. 2017; 8:1311.161. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016; 12:239–43.
Article162. Young KM, Gray CM, Bekker LG. Is obesity a risk factor for vaccine non-responsiveness? PLoS One. 2013; 8:e82779.
Article163. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013; 14:232–44.
Article164. Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015; 396:1181–97.
Article165. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–12.
Article166. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018; 9:754.
Article167. Viikari LA, Huupponen RK, Viikari JS, et al. Relationship between leptin and C-reactive protein in young Finnish adults. J Clin Endocrinol Metab. 2007; 92:4753–8.
Article168. Hribal ML, Fiorentino TV, Sesti G. Role of C reactive protein (CRP) in leptin resistance. Curr Pharm Des. 2014; 20:609–15.
Article169. Liu Y, Liu C, Jiang C, et al. C-reactive protein inhibits high-molecular-weight adiponectin expression in 3T3-L1 adipocytes via PI3K/ Akt pathway. Biochem Biophys Res Commun. 2016; 472:19–25.
Article170. Naylor C, Petri WA Jr. Leptin regulation of immune responses. Trends Mol Med. 2016; 22:88–98.
Article171. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017; 98:51–8.
Article172. Dayakar A, Chandrasekaran S, Veronica J, Maurya R. Leptin induces the phagocytosis and protective immune response in Leishmania donovani infected THP-1 cell line and human PBMCs. Exp Parasitol. 2016; 160:54–9.173. Baltodano-Calle MJ, Polo-Vasquez JS, Romani-Pozo A, GutarraSaldana D, Guija-Poma E. Leptin as a potential prognostic marker of the severity of COVID-19 infection in obese patients. Nutr Metab Cardiovasc Dis. 2022; 32:743–4.
Article174. Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021; 42:2270–9.
Article175. Giner-Galvan V, Pomares-Gomez FJ, Quesada JA, et al. C-Reactive protein and serum albumin ratio: a feasible prognostic marker in hospitalized patients with COVID-19. Biomedicines. 2022; 10:1393.
Article176. Tonon F, Di Bella S, Giudici F, et al. Discriminatory value of adiponectin to leptin ratio for COVID-19 pneumonia. Int J Endocrinol. 2022; 2022:9908450.
Article177. van der Voort PHJ, Moser J, Zandstra DF, et al. Leptin levels in SARS-CoV-2 infection related respiratory failure: a cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon. 2020; 6:e04696.
Article178. Larsson A, Lipcsey M, Hultstrom M, Frithiof R, Eriksson M. Plasma leptin is increased in intensive care patients with COVID-19: an investigation performed in the PronMed-Cohort. Biomedicines. 2021; 10:4.
Article179. Blot M, David M, Nguyen M, et al. Are adipokines the missing link between obesity, immune response, and outcomes in severe COVID-19? Int J Obes (Lond). 2021; 45:2126–31.
Article180. Wang J, Xu Y, Zhang X, et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J Leukoc Biol. 2021; 110:9–20.
Article181. Honce R, Schultz-Cherry S. A tale of two pandemics: obesity and COVID-19. J Travel Med. 2020; 27:taaa097.
Article182. Brandao SCS, Godoi E, de Oliveira Cordeiro LH, et al. COVID-19 and obesity: the meeting of two pandemics. Arch Endocrinol Metab. 2021; 65:3–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Innate Immunity in Diabetes and Metabolism: Recent Progress in the Study of Inflammasomes
- Alterations in Gut Microbiota and Immunity by Dietary Fat
- Inflammatory Receptors/Ligands as a Novel Target Against Obesity-Induced Inflammation and Metabolic Diseases
- Association between Obesity and Infection
- Non-nutritive Sweeteners and Their Associations with Obesity and Type 2 Diabetes